Biomarkers for Myocardial Infarction and Chronic Heart Failure - CAM 193
Description
Cardiac biomarkers are the biochemical markers released in blood from injured myocardial tissue in both acute and chronic conditions, such as myocardial infarction or heart failure. They become elevated in blood after a certain period and can be measured. Examples of cardiac biomarkers commonly used in the acute clinical setting include troponin and creatine kinase MB isoenzyme (CKMB).1 Others, such as suppression of tumorigenicity 2 (ST2), can serve in long-term as markers of cardiomyocyte stress and fibrosis for risk stratification of patients with a wide spectrum of cardiovascular diseases.2
Regulatory Status
Many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). LDTs are not approved or cleared by the U.S. Food and Drug Administration; however, FDA clearance or approval is not currently required for clinical use.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- For individuals presenting with signs and symptoms of acute coronary syndrome (see Note 1), quantitative measurement of cardiac troponin (troponin T or I) for the diagnosis of myocardial infarction (MI) (when tested at an outpatient facility capable of performing an adequate clinical MI evaluation) is considered MEDICALLY NECESSARY up to four times within the first 72 hours following initial presentation.
- Measurement of B-type natriuretic peptide (BNP) or N-terminal proBNP (NT-proBNP) is considered MEDICALLY NECESSARY in any of the following situations:
- To diagnose heart failure in individuals presenting with dyspnea.
- To establish disease severity in individuals with chronic heart failure (up to four times per year in the outpatient setting).
- For individuals presenting with signs and symptoms of acute coronary syndrome (see Note 1), measurement of following cardiac biomarkers for the diagnosis and/or prognosis of MI is considered NOT MEDICALLY NECESSARY:
- Aspartate aminotransferase (AST/SGOT).
- Cardiac creatine kinase isoenzyme MB (CKMB).
- Creatine kinase (CK).
- Creatine kinase isoenzymes.
- Lactate dehydrogenase (LD, LDH).
- Myoglobin.
- For individuals presenting with signs and symptoms of acute coronary syndrome (see Note 1), measurement of cardiac biomarkers in an outpatient setting which is not capable of performing adequate clinical MI evaluation (e.g., independent lab or physician’s office) is considered NOT MEDICALLY NECESSARY.
- In the outpatient setting, qualitative measurement of cardiac troponin (troponin T or I) is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of an individual’s illness.
- For individuals presenting with signs and symptoms of acute coronary syndrome (see Note 1), measurement of the following cardiac biomarkers for the diagnosis and/or prognosis of MI is considered NOT MEDICALLY NECESSARY:
- Copeptin
- Troponin C
- C-reactive protein
- Heart-type fatty acid binding protein (H-FABP)
- Any other cardiac biomarkers not listed above
- For all situations in the outpatient setting, analysis of ST2 and/or its isoforms (e.g., Presage ST2) is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: Acute Coronary Syndrome/Myocardial Infarction Common Signs and Symptoms:3
- Ischemic chest pain with radiation to an upper extremity, radiation to both arms, and pain associated with diaphoresis or with nausea and vomiting.
- Squeezing, tightness, pressure, constriction, crushing, strangling, burning, heartburn, fullness in the chest, band-like sensation, knot in the center of the chest, lump in throat, ache, heavy weight on chest and toothache (when there is radiation to the lower jaw).
- Ischemic pain often radiates to other parts of the body including the upper abdomen (epigastrium), shoulders, arms (upper and forearm), wrist, fingers, neck and throat, lower jaw and teeth (but not upper jaw), and not infrequently to the back (specifically the interscapular region).
- Shortness of breath, belching, nausea, indigestion, vomiting, diaphoresis, dizziness, lightheadedness, clamminess, and fatigue.
Atypical Signs and Symptoms:3
Dyspnea alone, weakness, nausea and/or vomiting, epigastric pain or discomfort, palpitations, syncope, or cardiac arrest.
Table of Terminology
| Term |
Definition |
| AATS |
American Association for Thoracic Surgery |
| ACC |
American College of Cardiology |
| ACS |
Acute coronary syndrome |
| ADH |
Antidiuretic Hormone |
| AHA |
American Heart Association |
| AMI |
Acute myocardial infarction |
| APACE |
Advantageous predictors of acute coronary syndrome evaluation |
| ASCP |
American Society for Clinical Pathology |
| ASE |
American Society of Echocardiography |
| ASNC |
American Society of Nuclear Cardiology |
| AST |
Aspartate aminotransferase |
| AUC |
Appropriate use criteria |
| AUC |
Area under the curve |
| AVP |
Arginine vasopressin |
| B |
Brain |
| BB |
Bundle branch |
| BMI |
Body mass index |
| B-NR |
Level B – nonrandomized |
| BNP |
Brain natriuretic peptide |
| CABG |
Coronary artery bypass grafting |
| CCS |
Canadian Cardiovascular Society |
| CHOPIN |
Copeptin Helps in the Early Detection of Patients with Acute Myocardial Infarction |
| CK |
Creatine kinase |
| CKMB |
Creatine Kinase MB isoenzyme |
| CKMM |
Creatine kinase – skeletal muscle |
| CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CMP |
Cardiomyopathy |
| CMS |
Centers For Medicare and Medicaid |
| CPK |
Creatine phosphokinase |
| cTnI |
Cardiac troponin I |
| cTnT |
Cardiac troponin T |
| ECG |
Electrocardiogram |
| ED |
Emergency Department |
| ESC |
European Society of Cardiology |
| FDA |
Food and Drug Administration |
| GDF-15 |
Growth differentiation factor 15 |
| HCPCS |
Healthcare common procedure coding system |
| H-FABP |
Heart-type fatty acid binding protein |
| HF |
Heart failure |
| HFSA |
Heart Failure Society of America |
| hs-cTn |
High-sensitivity cardiac troponin |
| IL-1R |
Interleukin 1 receptor |
| IL-2 |
Interleukin 2 |
| IL-33 |
Interleukin 33 |
| ILCOR |
International Liaison Committee on Resuscitation |
| LAMP |
Leicester acute myocardial infarction peptide gene |
| LBBB |
Left bundle branch block |
| LD or LDH |
Lactate dehydrogenase |
| LDT |
Laboratory-developed test |
| LOE |
Level of evidence |
| M |
Muscle |
| MACE |
Major adverse cardiovascular events |
| MI |
Myocardial infarction |
| MINOCA |
Myocardial infarction in the absence of obstructive coronary artery |
| NICE |
National Institute for Health and Care Excellence |
| NSTE-ACS |
Non-ST-elevation acute coronary syndromes |
| NSTEMI |
Non-ST-elevation myocardial infarction |
| NT-proBNP |
N-terminal pro-B-type natriuretic peptide |
| PA |
Prior authorization |
| PCI |
Percutaneous coronary intervention |
| POC |
Point-of-care |
| ROC |
Receiver operating characteristic |
| SCAI |
Society for Cardiovascular Angiography and Interventions |
| SCCT |
Society for Cardiovascular Computed Tomography |
| SGOT |
Serum glutamic-oxaloacetic transaminase |
| sST2 |
Soluble suppression of tumorigenicity 2 |
| ST2 |
Soluble interleukin 1 receptor-like 1 |
| ST2L |
Transmembrane isoform of S2 |
| STEMI |
ST-elevation myocardial infarction |
| STS |
Society of Thoracic Surgeons |
| ST-T |
ST-segment-T Wave |
| T |
Troponin |
| TIMI |
Thrombolysis in myocardial infarction |
| TnC |
Troponin C |
| TnI |
Troponin I |
| TnT |
Troponin T |
| ULN |
Upper limit of normal |
| URL |
Upper reference limit |
| WHF |
World Heart Federation |
| WHO |
World Health Organization |
Rationale
Acute coronary syndromes (ACS) represent continuous events starting with angina, reversible injury, and progressing to unstable angina; these syndromes are frequently associated with minor myocardial damage, and myocardial infarction (MI) that results in extensive tissue necrosis.1 Patients with ACS usually present with chest pain and associated signs and symptoms. These patients are subdivided into two major categories based on the 12-lead electrocardiogram (ECG). If an ST-segment elevation is observed on the ECG, it is indicative of acute ST-elevation myocardial infarction (STEMI) type of ACS. If the ECG shows ST-segment depression, T wave changes, or no ECG abnormalities, it is indicative of non-ST-elevation myocardial infarction (NSTEMI) and unstable angina.
Acute coronary syndromes are complex. However, the most common cause is atherosclerotic coronary artery disease with rupture of atherosclerotic plaque.4 The first documented definition of acute MI was established in 1979 by the World Health Organization (WHO). It included in the criteria for MI diagnosis the recommendation to use the rise or fall patterns of cardiac biomarkers, such as creatine kinase (CK), creatine kinase’s MB isoenzyme (CKMB), lactate dehydrogenase (LDH) or aspartate aminotransferase (AST) activities.5 Since then, other societies have proposed their own criteria for diagnosis. The third universal definition of MI includes typical clinical symptoms, suggestive ECG changes, or imaging evidence of new loss of viable myocardium or new regional wall abnormality with a rise and/or fall of cardiac biomarkers.6 Nonetheless, the universal criteria are being refined by cardiovascular societies and will likely change with scientific progress and better understanding of MI pathophysiology.
Myocardial infarction (MI) results in cardiac injury and extensive tissue necrosis. The cellular membranes become compromised and release structural proteins and other macromolecules into cardiac interstitial space. These released markers are called cardiac biomarkers. The levels of
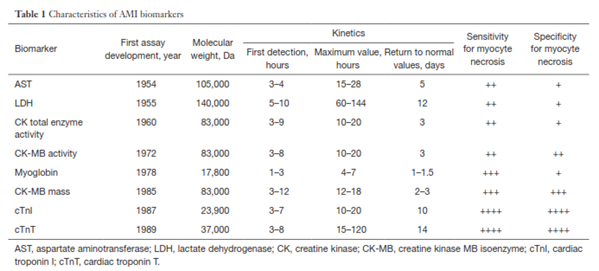
these cardiac biomarkers in blood will rise and fall with time after MI.1 The first cardiac biomarker, aspartate aminotransferase (AST), was used for MI diagnosis in 1954. AST is present in human tissues as two isoenzymes: cytoplasmic and mitochondrial. AST is a non-specific biomarker, and its activity could also be elevated in other conditions, such as hepatic congestion secondary to congestive heart failure. Since then, other cardiac biomarkers were used as an aid in diagnosis of MI, but due to their non-specificity and other reasons, many of them are no longer used in clinical practice or their use remains very limited.7 The most common cardiac biomarkers and their characteristics are summarized in Table 1 from Danese and Montagnana (2016).
Heart failure (HF) is a complex clinical syndrome resulting from any structural or functional impairment of ventricular filling or ejection of blood, including disorders of the pericardium, myocardium, endocardium, heart valves, great vessels, or certain metabolic abnormalities.8 Most patients with HF have symptoms due to impaired left ventricular (LV) myocardial function.9,10 The most common symptoms of HF are dyspnea and fatigue, which may limit exercise tolerance and fluid retention. Some patients have exercise intolerance but little evidence of fluid retention, whereas others complain primarily of edema, dyspnea, or fatigue.9 HF is often a progressive condition, beginning with predisposing factors and leading to the development and worsening of clinical illness.9,11
Lactate Dehydrogenase (LDH, also known as LD)
Lactate dehydrogenase is a cytoplasmic enzyme present in many different tissues, such as skeletal muscle, liver, heart, kidney, and red blood cells. Five isoenzymes have been identified by gel electrophoresis and other techniques.12 The heart isoenzymes, LD1 and LD2, have activity increases in blood five to ten hours after MI symptoms onset and remains elevated for up to ten days.7 LD has poor specificity for cardiac tissue and is generally not recommended as a biomarker for the diagnosis of MI.4,13
Myoglobin
Myoglobin is an oxygen-binding, cytoplasmic, heme protein. It is one of the first cardiac biomarkers measurable in the serum that appears between one and three hours after MI symptoms onset. Myoglobin is present in skeletal and cardiac muscles and is cleared by the kidneys. Its clinical utility is limited by its poor specificity. The main reason of using myoglobin in a clinical setting was its sensitivity for MI:7 but with appearance of sensitive troponin assays, myoglobin use offers little advantage for the diagnosis of MI.14,15 Currently, there are no recommendations for myoglobin to be used in the diagnosis of MI,4 and its use as cardiac biomarker is discouraged.4,13
Creatine Kinase (CK) Isoenzymes and Isoform MB (CKMB)
The cytosolic enzyme CK, formerly known as creatine phosphokinase,7 is present as three cytosolic isoenzymes and one mitochondrial isoenzyme. These isoenzymes are dimers of M (muscle) and B (brain) chains that exist in three combinations: MM, MB and BB.16 The CKMM is predominant in both heart and skeletal muscle, but CKMB is more specific for the myocardium. The total CK activity could be detected in blood three to nine hours after MI, but it reaches the maximum levels in blood in 10-20 hours and returns to normal in about 72 hours.17 The measurement of total CK activity is not specific to MI because it also increases in liver, biliary tract, kidneys, and skeletal muscle disease, and its measurement is problematic in older individuals with lower muscle mass.18-20 CKMB mass (CKMB protein concentration measurements) was once the cardiac biomarker of the choice that replaced CK, CKMB activity, AST, and LDH.7 However, with arrival of cardiac troponin assays, the use of CKMB became less popular. Some clinicians advocate for the use of CKMB for diagnosis and prognosis of MI, but cardiac troponins have shown either equally reliable or superior results compared to CKMB; consequently, troponin is the recommended test for MI diagnosis now.4,13
Engel and Rockson (2020) studied the use of CKMB in early diagnosis of MI within the first nine hours of the hospital stay. The authors studied 528 patient charts of patients who had complained of chest pain. An enzymatic diagnosis was assigned if CKMB exceeded the normal values. The diagnosis of each patient before nine hours (early diagnosis) was compared to the ultimate diagnosis at 14-24 hours (final diagnosis). Of the 528 patients, 195 (36.9%) had an early MI diagnosis within nine hours and 190 patients (97.4%) of these did have an ultimate diagnosis of MI. Therefore, the authors conclude that "standard CK-MB measurements within nine hours of arrival provided an accurate clinical assessment in > 99% of the cases.”21
Troponins
The regulatory protein troponin in the troponin complex is composed of three isoforms. Troponin C (TnC) is responsible for calcium binding and has no role to play as a cardiac biomarker. Troponin I (TnI) and Troponin T (TnT) are responsible for inhibition of ATPase activity and tropomyosin binding, respectively.22 Contrary to all previously used cardiac biomarkers, cardiac troponins have high specificity for cardiac tissue. The cardiac troponins have a specific pattern of expression because they have different amino sequences encoded by different genes for skeletal and cardiac muscles. Cardiac TnI has an additional 31-amino acid residue compared to skeletal muscle. This protein is not expressed in normal, regenerating, or diseased skeletal muscle from human or animal origin.23 Cardiac TnT has an additional 11-amino acid residue, but this protein was also found in regenerating rat skeletal muscle, during human fetal development, and in diseased human skeletal muscle.24-26 In addition, cardiac TnT was also found in skeletal muscle specimens from patients with muscular dystrophy, polymyositis, and chronic renal disease.24,27
Neumann, et al. (2019) evaluated high-sensitivity troponin (troponin I and T)’s ability to predict MI and subsequent 30-day outcomes. The authors developed a risk assessment tool based on patients presenting to the emergency department with “symptoms suggestive of myocardial infarction.” Concentrations of troponin I or T were measured at presentation and after early or late serial sampling. Cutoffs were then determined to create cutoffs for risk assessment. Among the 22651 patients (9604 in derivation cohort, 13047 in validation cohort), the total prevalence of MI was 15.3%. The authors found that “lower high-sensitivity troponin concentrations at presentation and smaller absolute changes during serial sampling were associated with a lower likelihood of myocardial infarction and a lower short-term risk of cardiovascular events.”28
Anand, et al. (2019) evaluated the adoption rate of the universal definition of MI and the corresponding recommendations. A total of 1902 medical centers over 23 countries were surveyed, and the authors obtained answers regarding the primary biomarker, diagnostic thresholds, and clinical pathways used to identify MI. The authors found that cardiac troponin was the primary biomarker used at 96% of surveyed sites, with 41% of these sites using high-sensitivity troponins. The sites using high-sensitivity assays were also more likely to use serial sampling (91% vs 78% using “contemporary” sensitivity troponin) and the 99% percentile diagnostic threshold (74% vs 66%). Use of creatine kinase MB (CKMB) was “very limited” outside of Latin America.29
In addition, other cardiac biomarkers, such as heart-type fatty acid binding protein (H-FABP) and copeptin, have been reported in the scientific literature. However, they are not commonly used in clinical settings.13
Boeddinghaus, et al. (2020) compared the diagnostic accuracy of high-sensitivity cardiac troponin (hs-cTn) TriageTrue assay in patients with suspected MI with other laboratory assays including Elecsys cTnT-hs assay and hs-cTnI-Architect assay. A total of 1,261 patients with patients suggestive of MI were enrolled in the study. The TriageTrue assay ruled out patients with troponin I concentration less than three ng/l and classified these patients as low-risk of MI and ruled in patients with a troponin I concentration > 60 ng/l. Out of the 1,261 patients enrolled in the study, 178 were diagnosed with MI based on troponin I levels of > 60 ng/l using the TriageTrue assay. TriageTrue troponin I concentrations were higher in patients with MI than in patients with other final diagnoses. Other diagnosis included unstable angina in 13 of 1,261 (nine percent), tachyarrhythmia, Takotsubo syndrome, HF, or myocarditis in 208 patients (17%), and noncardiac symptoms in 714 patients (57%). The AUC of the TriageTrue assay was 0.95, the hs-cTnT-Elecsys assay AUC was 0.93, and hs-cTnI-Architect assay AUC was 0.92. The TriageTrue algorithm allowed providers to make a triage decision after one hour in 401 of 545 patients. The efficacy for rule-out or rule-in was 43% for the TriageTrue, 25% in f hs-cTnTElecsys, and 22% in hs-cTnI-Architect. Ruled-out patients had cumulative event rates of zero percent at 30 days and 1.6% at two years. Overall, the authors conclude that “POC-hs-cTnI-TriageTrue assay provides high diagnostic accuracy in patients with suspected MI with a clinical performance that is at least comparable to that of best-validated central laboratory assays.”30
Heart-type fatty acid binding protein (H-FABP)
Heart-type fatty acid binding protein, a small cytoplasmic protein present in cardiomyocytes, is believed to have a function in myocardial lipid homeostasis.31 Because of its small size, this protein appears in the blood after MI almost as early as myoglobin, but it has better specificity than myoglobin for cardiac tissue.32 Seino, et al. (2003) compared the use of H-FABP with rapid troponin in 371 patients with acute chest pain. Their study demonstrated that H-FABP had significantly higher sensitivity (89%) than troponin T (22%) and myoglobin (38%), but it has lower specificity (52%) than troponin (94%). Other studies were performed to compare H-FABP to troponins; however, they were unable to demonstrate superior results compared to troponins. H-FABP is not encouraged for assessment of MI as troponins are generally superior.13
In a prospective, cross-sectional study, Nguyen, et al. (2020) studied the diagnostic utility of H-FABP in the early diagnosis of acute MI in comparison with troponin I and CKMB. A total of 216 patients enrolled in the study with 179 of those diagnosed with acute MI. H-FABP, CKMB, and troponin I levels were compared. H-FABP reached its highest concentration in 6-12 hours after symptoms of chest pain, with a mean value of 169 ng/mL in acute MI patients. The cutoff value was 5.7 ng/ml with 90.5% sensitivity and 100% specificity. The combination of H-FABP, CKMB and troponin I together had the highest sensitivity of 97.2%. The AUC of H-FABP was observed to be 0.99, which was higher than CKMB (0.92) and troponin I (0.86). The authors conclude that "H-FABP can be used as a reliable diagnostic cardiac biomarker in the early detection of AMI for patients who came to the emergency room within 12h of onset of chest pain.”34
Copeptin
Copeptin is the 39 amino acid C-terminal fragment cleaved from pro-arginine vasopressin (AVP). After MI, copeptin levels increase rapidly and decline over the next two to five days.35 In the Copeptin Helps in the Early Detection of Patients With Acute Myocardial Infarction (CHOPIN) 16-site study involving 1,967 patients presenting within six hours of pain onset, copeptin was shown to have a potential value in ruling out MI with a negative predictive value greater than 99% when combined with TnI measurements.36 The Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) multicenter study, involving 1,439 patients presenting with MI symptoms, demonstrated no benefit in using copeptin as a an early rule-out cardiac biomarker for MI.37 Copeptin is not encouraged for assessment of MI as troponins are generally superior.13
ST2
Part of the interleukin-1 receptor family with two isoforms, ST2 has two isoforms: soluble ST2 (sST2) and ST2L. ST2 is the receptor of the IL-33 cytokine that can be secreted by living cells in response to cellular stress and mechanical strain. IL-33 binds the receptor complex of ST2L and IL-1R accessory protein and reduces myocardial fibrosis, prevents cardiomyocyte hypertrophy, reduces apoptosis, and improves myocardial function. The cardioprotective effects of IL-33 are specifically through the ST2L receptor. However, sST2 may also bind IL-33, blocking the interaction between IL-33/ST2L. This eliminates the cardioprotective effects of the IL-33/ST2L interaction.38 Experimentally, this leads to cardiac hypertrophy, fibrosis, and ventricular dysfunction.39
One of the main proprietary tests used to assess ST2 levels is the Presage Assay by Critical Diagnostics. This assay was approved by the FDA on December 9, 2011. According to the FDA, “The Critical Diagnostics Presage® ST2 Assay kit is an in vitro diagnostic device that quantitatively measures ST2 in serum or plasma by enzyme-linked immunosorbent assay in a microtiter plate format. The Presage® ST2 Assay is indicated to be used in conjunction with clinical evaluation as an aid in assessing the prognosis of patients diagnosed with chronic heart failure.” The manufacturer claims a measuring range of 3.1 ng/mL of soluble ST2 to 200 ng/mL, and the data based on 1100 samples supports this claim. These 1100 samples found coefficient of variation of under five percent, a linear curve, and a r2=0.99.40
B-type Natriuretic Peptide (BNP)
B-type natriuretic peptide plays a role in salt and water management as well as pressure regulation within the natriuretic peptide system. When the prohormone proBNP is cleaved, it produces BNP and N-terminal probnp; BNP is released mostly from the left ventricle in the heart. An increase in the release of BNP may be indicative of HF and rapid measurement can establish or exclude the diagnosis of HF in patients with acute dyspnea.
A number of clinical assays are available for plasma BNP. These range from rapid point-of-care tests to lab tests that provide precise values for BNP. An NT-proBNP concentration greater than 900 pm/mL is “roughly” the same as a BNP concentration that is greater than 100 pg/mL.41
Natriuretic peptide biomarkers should be measured in patients who present with dyspnea to diagnose HF, but these biomarkers must be considered as part of a complete patient evaluation and not used in isolation. For prognosis, natriuretic peptide biomarkers can be used in patients with chronic HF and used when patients are admitted to the hospital with acutely decompensated HF. Lastly, there may be value in measuring natriuretic peptide biomarkers predischarge from the hospital.41
N-terminal pro-B-type Natriuretic Peptide (NT-proBNP)
Measurement of NT-proBNP is of value in diagnosis and prognosis of HF and other cardiovascular diseases. Studies show that the accuracy of diagnosing HF across various settings improves with measurement of NT-proBNP values. Like BNP, NT-proBNP is helpful when used with patients presenting with dyspnea. The “optimal” measurement value for differentiating between HF and other causes of dyspnea varies with patient age.
B-type natriuretic peptide and N-terminal probnp both fall in concentration after effective therapeutic treatment of chronic HF, which means that serial measurements have shown some promise in therapeutic management. However the effectiveness and use of serial BNP measurements in monitoring patient response to acute HF treatment is still under investigation.41
Proprietary Testing
Proprietary tests for various biomarkers are available in several clinical settings. Platforms including Roche’s “CARDIAC Trop T Sensitive test” and Responsebio’s battery of cardiac tests emphasize their speed (on the scale of minutes) and versatility.42,43
No single diagnostic test for HF exists because it is a clinical diagnosis based on a careful history and physical examination. However, biomarkers of cardiovascular diseases have been developed for diagnosis and prognosis, and the use of several biomarkers is now considered the standard of care. ST2 is a marker of cardiomyocyte stress and fibrosis that adds additional value to natriuretic peptides, resulting in a risk stratification of patients with a wide spectrum of cardiovascular diseases.2
Clinical Utility and Validity
Jeong, et al. (2020) studied the diagnostic value of copeptin for early diagnosis of acute MI in comparison with troponin I and CKMB. There were 271 patients complaining of chest pain within six hours of onset were studied within the emergency department. The diagnostic performance of copeptin, troponin I, and CKMB was compared by assessing the AUC and ROC curve analysis. After comparing AUC, copeptin had a significantly better diagnosis value than troponin I in patients with chest pain within two hours of onset. In addition, troponin I and copeptin together had better diagnostic performance than CKMB and troponin I combination. Overall, the authors conclude that "the combination of troponin I and copeptin improves AMI diagnostic performance in patients with early-onset chest pain in an ED setting.”44
Ky, et al. (2011) conducted a multicenter prospective study to evaluate whether plasma ST2 levels predict adverse outcomes in 1,141 chronic HF outpatients. Patients in the highest ST2 tertile (ST2 > 36.3 ng/mL) had a “markedly increased” risk (hazard ratio 3.2) of adverse outcomes compared to the lowest tertile ≤ 22.3 ng/mL). The investigators concluded that “ST2 is a potent marker of risk in chronic heart failure and when used in combination with NT-proBNP offers moderate improvement in assessing prognosis beyond clinical risk scores.”45
Wang, et al. (2012) studied the prognostic value of three novel biomarkers induced by cardiovascular stress. The investigators measured sST2, growth differentiation factor-15, and high-sensitivity troponin I in 3,428 participants in the Framingham Heart Study. Multivariable-adjusted proportional hazards models were performed to assess the individual and combined ability of the biomarkers to predict adverse outcomes. The three new biomarkers were associated with death, major cardiovascular events, and HF, but not with coronary events. The investigators concluded that the findings demonstrated the prognostic value of the newer biomarkers in healthy individuals.46
Wijk, et al. (2014) provided a follow-up on the largest study of long-term results of intensified NT-proBNP-guided versus symptom-guided management of elderly patients with HF. The TIME-CHF study randomized 499 patients with HF that were ages 60 and older with LV ejection fraction; patients were provided either guided NT-proBNP treatment or symptom-guided therapy over a period of 18 months. The results of the study showed “NT-proBNP–guided therapy did not improve the primary end point compared with symptom-guided therapy but did improve HF hospitalization-free survival.”47
Wang, et al. (2018) investigated the possibility of using sST2 as a biomarker to distinguish between acute aortic dissection and other causes of acute chest pain. Using an R&D Systems assay to measure plasma concentrations of sST2 in 1360 patients with a cutoff of 34.6 ng/mL, the researchers found that “sST2 had a sensitivity of 99.1%, specificity of 84.9%, positive predictive value of 68.7%, negative predictive value of 99.7%, positive likelihood ratio of 6.6, and negative likelihood ratio of 0.01.” Additionally, within 24 hours of symptom onset, sST2 levels were higher in those with acute aortic dissection in comparison to those with acute MI or pulmonary embolism. sST2 was also superior in overall diagnostic performance to D-dimer and troponin I using the area under receiver operating characteristic curves.
Januzzi, et al. (2013) conducted a retrospective study to assess sST2 as a prognostic marker after orthotopic heart transplantation (OHT) and as a test to predict acute cellular rejection. sST2 concentrations were measured in 241 patients following OHT. Elevated sST2 was associated with cellular rejection, with highest rates of cellular rejection in the fourth sST2 quartile. No significant association between sST2 and antibody-mediated rejection or allograft vasculopathy was found. A sST2 level of ≥30 ng/mL was found to independently predict death over the 7-year follow-up with a hazard ratio of 2.1. The investigators concluded that sST2 levels are associated with the presence of cellular rejection and predict long-term mortality following OHT.49
Boman, et al. (2018) assessed the prognostic value of ST2 on cardiovascular mortality. A total of 159 patients were evaluated, but ST2 was not found to be significantly associated with cardiovascular mortality or all-cause mortality. Furthermore, no significant interaction of ST2 and N-terminal prohormone of brain natriuretic peptide /N-terminal pro-BNP was found.50
Dimitropoulos, et al. (2020) investigated the association of soluble suppression of tumorigenesis-2 (sST2) with endothelial function in patients with ischemic HF. A total of 143 patients with “table HF of ischemic etiology and reduced left ventricular ejection fraction (LVEF)” were included along with 77 controls. The authors found an increased level of sST2 in HF patients compared to controls (15.8 ng/mL compared to 12.5 ng/mL). Within the HF group, there was no association of LVEF with sST2. Overall, sST2 levels were found to be increased and associated with functional capacity in “patients with chronic HF of ischemic etiology.” Finally, the authors found an inverse association between flow-mediated dilation and sST2 levels, which the authors stated “highlight[ed] the interplay between the dysfunctional endothelium and HF pathophysiologic mechanisms.”51
Hou, et al. (2020) aimed to investigate the association between sST2 levels and clinical outcomes of high-risk HF. The primary endpoint was defined as all-cause mortality. A total of 150 patients were included; all-cause mortality occurred in 16 of the patients over the course follow-up. The authors found that all-cause mortality increased significantly above 34.98846 ng/mL by a factor of 16% to 5.33%. After adjusting the model for certain co-factors (age, gender, et al.), and after adding NT-proBNP, “the risk of all-cause death was increased by 2.5% and 1.9%, respectively, per ng/ml of sST2.” The authors identified the best sST2 cutoff for predicting all-cause mortality to be 43.42671 ng/ml, with an area under the curve of 0.72, sensitivity of 0.69, and specificity of 0.69. Risk of all-cause mortality was found to be 21.2% above this cutoff and 5.1% below it, with a corresponding hazard ratio of 3.30. The authors concluded that “Patients with sST2 levels more than 43.42671 ng/ml even after ICD implantation should therefore be monitored carefully.”52
Heart failure has been recognized as a common complication of diabetes, with a prevalence of up to 22%. Data also suggest that HF may develop in individuals with diabetes even in the absence of hypertension, coronary heart disease, or valvular heart disease and, as such, represents a major cardiovascular complication in this vulnerable population.53 Jarolim, et al. (2018) investigated the prognostic implications of changes in N-terminal BNP (NT-proBNP) concentration in patients with type 2 diabetes and ischemic heart disease. The study observed a strong graded relationship between increasing baseline and 6-month NT-proBNP concentration and the incidence of major CV events, in particular hospitalization for HF among diabetic individuals.54
American Diabetes Association (ADA)
In the 2025 standards of care in diabetes the ADA has a section on cardiovascular disease and risk management. The ADA calls out that adults with diabetes are at increased risk for the development of asymptomatic cardiac structural or functional abnormalities (stage B HF) or symptomatic (stage C) HF. As such, the ADA recommends to consider screening adults with diabetes by measuring a natriuretic peptide (BNP or NT-proBNP) to facilitate prevention of stage C HF.55
In a consensus report from 2022, the ADA provides recommendations for early detection of subclinical and stage B HF: “Specific to individuals with diabetes, measurement of natriuretic peptides (B-type natriuretic peptide [BNP]; N-terminal pro-BNP [NT-proBNP]) or high-sensitivity cardiac troponin is particularly helpful to identify stage B HF and predict progression to symptoms or death from HF. . . Furthermore, while one natriuretic peptide or troponin measurement may provide important prognostic insights, serial measurements to detect rising values of either increase sensitivity for identifying those at highest risk for incident HF. As an example, in individuals with T2D in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) trial, two NT-proBNP measurements spaced 6 months apart were able to identify those at highest risk (both elevated), rising risk (baseline low, follow-up higher), or lower risk (6-month measurement lower). . . Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest HF stages and implement strategies to prevent transition to symptomatic HF. This recommendation is based on the substantial data indicating the ability of these biomarkers to identify those in stage A or B at highest risk of progressing to symptomatic HF or death, together with evidence that the risk in such individuals may be lowered through targeted intervention or multidisciplinary care.”53
2018 ESC/ACC/AHA/WHF Fourth Universal Definition of Myocardial Infarction
Both cTnI and cTnT are recommended for evaluation of myocardial injury, and high-sensitivity cTn assays are recommended for routine clinical use. An acute MI is designated when a rising/falling pattern is seen with cTn levels and if there is at least one measurement greater than the 99th percentile of the upper reference limit (URL).56
Creatine Kinase MB isoenzyme is considered less sensitive and specific than either troponin. However, in the absence of a cTn assay, CKMB is considered the best alternative. A measurement of CKMB above the 99th percentile of the URL should be “designated as the decision level for the diagnosis of MI.” Sex-specific CK-MB values should be used.56
In the 2019 AHA guideline discussing the “Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease [MINOCA],” the AHA notes that the diagnostic criteria of MINOCA follows the “Fourth Universal Definition of Myocardial Infarction” above, specifically the rise or fall of cardiac troponin levels with at least one value above the 99th percentile of the reference limit. The guideline considers this definition “fundamental” to identifying and defining MINOCA.57
2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes (NSTE-ACS)
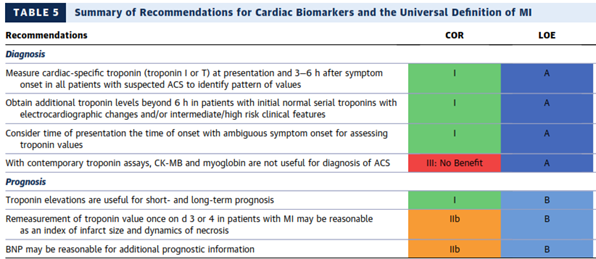
The American College of Cardiology (ACC) and the American Heart Association (AHA) have developed clinical practice guidelines to provide recommendations applicable to patients with or at risk of developing cardiovascular disease and to provide guidance to clinicians on optimal management of patients with NSTE-ACS. In their comprehensive document, the AHA/ACC panel has provided recommendations for initial evaluation and management of patients presenting with ACS symptoms, for the early hospital care, myocardial revascularization, late hospital care, hospital discharge and posthospital discharge care, special patient groups and quality of care and outcomes for ACS. The Task Force recommended to stratify patients with suspected ACS based on the likelihood of ACS and those with high-risk features should be referred immediately to the emergency department (ED). They have provided specific recommendations for the use of cardiac biomarkers in the diagnosis and prognosis of MI. They specifically recommended using troponin (troponin I or T when contemporary assay is used) for the diagnosis of MI. According to AHA/ACC guidelines, the cardiac troponin is recommended and should be measured at presentation and three to six hours after symptom onset in all patients who present with ACS symptoms. The panelists recommended identifying rising and/or falling pattern of troponin. In addition, they recommended measuring troponin levels beyond six hours after symptom onset in patients with normal troponins on serial examination when ECG changes and/or clinical presentation suggests ACS. If the onset of symptoms is not clearly identified, they recommended using the time of presentation as the time of onset for measuring troponin. The AHA/ACC guideline clearly highlighted that CKMB, or myoglobin should not be used for the diagnosis of ACS. All recommendations for the use of cardiac biomarkers in the diagnosis of MI were level A evidence.
The AHA/ACC guideline considered all recommendations in the use of cardiac biomarkers for ACS prognosis as level of evidence B. They considered the presence and magnitude of troponin elevations useful for short- and long-term prognosis. The re-measurement of troponin once on day three or four in patients with MI was considered reasonable to estimate the infarct size and dynamics of necrosis. Finally, they considered the use of BNP to be reasonable for additional prognostic information.
The recommendations for the use of cardiac biomarkers in the diagnosis and prognosis of MI was well summarized in Table 5 from 2014 AHA/ACC guidelines p.2655:4

Society for Cardiovascular Angiography and Interventions (SCAI)
In their expert consensus document titled “Consideration of a New Definition of Clinically Relevant Myocardial Infarction After Coronary Revascularization,” the SCAI expert panel introduced a new definition of clinically relevant MI after coronary revascularization percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). In their definition of clinically relevant MI after both PCI and CABG procedures, authors gave recommendations according to three different types of clinical presentation. In the first case, when patient has a normal CKMB baseline: “The peak CK-MB measured within 48 hours of the procedure rises to >10x the local laboratory ULN, or to >5x ULN with new pathologic Q-waves in >2 contiguous leads or new persistent LBBB, OR in the absence of CK-MB measurements and a normal baseline cTn, a cTn (I or T) level measured within 48 hours of the PCI rises to >70x the local laboratory ULN, or >35x ULN with new pathologic Q-waves in >2 contiguous leads or new persistent LBBB.” In the case when patients have elevated baseline CKMB (or cTn) with stable of falling biomarkers levels, they issued the following recommendation: “The CK-MB (or cTn) rises by an absolute increment equal to those levels recommended above from the most recent pre-procedure level.” And, in patients with elevated CKMB (or cTn), but without stable or falling biomarkers level, the recommendation was: “The CK-MB (or cTn) rises by an absolute increment equal to those levels recommended above plus new ST-segment elevation or depression plus signs consistent with a clinically relevant MI, such as new onset or worsening heart failure or sustained hypotension.” The authors have expressed preference to use CKMB instead of cTn, but they have included cTn in their definition if CKMB was not available.58
2015 AHA Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care
In their review of previously issued guidelines, the expert panel introduced new recommendations for diagnostic interventions in ACS regarding cardiac biomarkers. They still recommended to use Troponin in following situations: “We recommend against using hs-cTnT and cTnI alone measured at zero and two hours (without performing clinical risk stratification) to identify patients at low risk for ACS (Class III: Harm, LOE B-NR). We recommend that hs-cTnI measurements that are less than the 99th percentile, measured at zero and two hours, may be used together with low-risk stratification (TIMI score of zero or one or low risk per Vancouver rule) to predict a less than one percent chance of 30-day MACE (Class IIa, LOE B-NR). We recommend that negative cTnI or cTnT measurements at zero and between three and six hours may be used together with very low-risk stratification (TIMI score of zero, low-risk score per Vancouver rule, North American Chest Pain score of zero and age less than 50 years, or low-risk HEART score) to predict a less than one percent chance of 30-day MACE (Class IIa, LOE B-NR).” They did not express a preference in cardiac biomarkers to use, nor did they give any recommendations regarding CKMB.59
American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA)
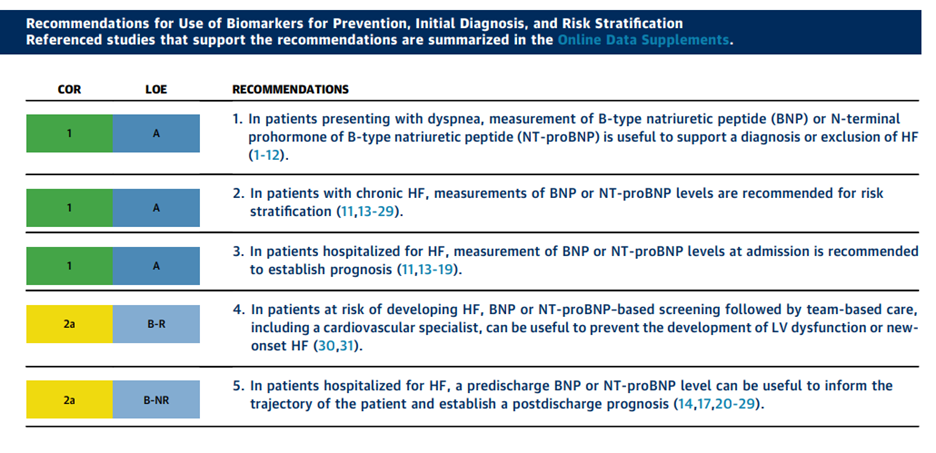
In 2017, the ACC/AHA/HFSA included information on BNP and NT-proBNP measurement for establishing prognosis or disease severity in chronic HF. Their recommendations:
- For prevention:
- “Class IIa recommendation (Level of Evidence: B-R) for utilizing natriuretic peptide biomarker-based screening for those at risk of developing HF, followed by team-based care including a cardiovascular specialist optimizing guideline-directed medical therapy (GDMT), to prevent the development of left ventricular dysfunction (systolic or diastolic) or new-onset HF.”
- For diagnosis:
- “Class I recommendation (Level of Evidence: A) for measurement of natriuretic peptide biomarkers in patients presenting with dyspnea, to support a diagnosis or exclusion of HF.”
- For prognosis or added risk stratification:
- “Class I recommendation (Level of Evidence: A) for measurement of B-type natriuretic peptide (BNP) or N-terminal (NT)-proBNP for establishing prognosis or disease severity in chronic HF.”
- “Class I recommendation (Level of Evidence: A) for measurement of baseline natriuretic peptide biomarkers and/or cardiac troponin on admission to the hospital to establish a prognosis in acutely decompensated HF.”
- “Class IIa recommendation (Level of Evidence: B-NR) for measurement of a predischarge natriuretic peptide level during a HF hospitalization, to establish a post-discharge prognosis.”
- “Class IIa recommendation (Level of Evidence: B-NR) for measurement of other clinically available tests, such as biomarkers of myocardial injury or fibrosis, in patients with chronic HF for additive risk stratification.”60
The full ACC/AHA article does not appear to support a standard of care that includes measuring BNP/NT-BNP for purposes of serial monitoring or therapeutic management, noting that “Because of the absence of clear and consistent evidence for improvement in mortality and cardiovascular outcomes, there are insufficient data to inform specific guideline recommendations related to natriuretic peptide-guided therapy or serial measurements of BNP or NT-proBNP levels for the purpose of reducing hospitalization or deaths in the present document.”60
In 2022, the ACC/AHA/HFSA updated their 2017 guideline on the management of heart failure. Regarding BNP and NT-proBNP assays, the authors emphasize that both tests can be used to establish the presence and severity of HF. However, they caveat that diagnostic sensitivity is impacted when a patient Is overweight – patients who are obese sometimes measure as having low levels of BNP and NT-proBNP.
Additional points of emphasis include:
- “A substantial evidence base supports the use of natriuretic peptide biomarkers for excluding HF as a cause of symptoms in ambulatory and emergency department settings.”
- “Although a reduction in BNP and NT-pro-BNP has been associated with better outcomes, the evidence for treatment guidance using serial BNP or NT-proBNP measurements remains insufficient.”
- “A widening array of biomarkers including markers of myocardial injury, inflammation, oxidative stress, vascular dysfunction, and matrix remodeling have been shown to provide incremental prognostic information over natriuretic peptides but remain without evidence of an incremental management benefit.”
The recommendations for the use of biomarkers was summarized in the table provided by the ACC/AHA/HFSA:61
American Heart Association (AHA)
The AHA notes sST2 as an “emerging” biomarker that supports diagnosis of HF with preserved ejection fraction, a biomarker that may predict mortality and HF events, and a biomarker that correlates with LV end-diastolic pressure. The AHA states that sST2 has numerous advantages as a biomarker, namely its concentration being unaffected by BMI, age, or renal function. SST2 is stated to correlate with HF prognosis as well. Overall, AHA states that out of the newer biomarkers (SST2, ST2, Gal-3, and GDF-15), “most appeal is driven by sST2.”62
A Scientific Statement published in 2019 also considered ST2 as the most “promising clinically” but also mentioned the limitations in consistency and utility in most inflammatory mediators. The Statement notes several clinical studies focusing on sST2 that are in progress as of March 24, 2020.63
European Society of Cardiology (ESC)
The ESC notes measurement of cardiac troponins as “mandatory” in all patients with suspected non-ST-elevation ACS. The guidelines assert that cardiac troponins are more sensitive and specific biomarkers of cardiomyocyte injury than CK, CKMB, and myoglobin. However, if troponin measurement is not possible, measurement of copeptin is recommended.
The ESC also acknowledges the natriuretic peptides (BNP, N-terminal pro-BNP and midregional pro-A-type natriuretic peptide) as providing useful prognostic information along with the troponins. The ESC mentions other biomarkers such as midregional pro-adrenomedullin, growth differentiation factor 15 and copeptin, but they cannot recommend them at this time as their added value in risk assessment seems “marginal.”64
The 2019 ESC guidelines focusing on chronic coronary syndromes states that for “clinical suspicion of coronary artery disease instability…management should follow the Guidelines for ACS without persistent ST-segment elevation,” which is discussed above.65
The 2020 ESC guidelines focus on diagnosis of acute coronary syndrome. Regarding MI, they recommend that “the routine use of copeptin as an additional biomarker for the early rule-out of MI should be considered where hs-cTn assays are not available.” In addition, “CK-MB shows a more rapid decline after MI and may provide added value for detection of early reinfarction.”66
The updated 2023 ESC Guidelines for the management of acute coronary syndromes concluded that “after excluding clinical and ECG signs suggestive of STEMI or very high-risk NSTE-ACS, biomarkers play a complementary role in the diagnosis, risk stratification, and management of patients with suspected ACS. Measurement of a biomarker of cardiomyocyte injury, preferably high-sensitivity cardiac troponin (hs-cTn), is recommended in all patients with suspected ACS.” As for other biomarkers ESC concludes “use of biomarkers other than cTn for the diagnosis of ACS is not recommended (unless cTn is not available). Among the multitude of additional biomarkers evaluated for the diagnosis of NSTEMI, only creatine kinase myocardial band isoenzyme, myosin-binding protein C, and copeptin may have clinical relevance when used in combination with (standard) cTn T/I, although in most clinical situations their incremental value above and beyond cTn is limited.”67
The 2024 ESC Guidelines for the management of chronic coronary syndromes reaffirmed the above statement by adding that if there is a clinical suspicion of coronary artery disease instability, “biochemical markers of myocardial injury—such as troponin T or troponin I—should be measured, preferably using high-sensitivity assays, and management should follow the 2023 ESC Guidelines for the management of patients with acute coronary syndromes.”68 The guidelines then go on to state that “while multiple biomarkers may be useful for prognostication, they do not yet have a role in diagnosing obstructive coronary artery disease, but some promising results have been published. Measuring NT-proBNP helps confirm or exclude suspected HF.”68
In their 2016 guidelines on acute and chronic heart failure, the ESC states that “although there is extensive research on biomarkers in HF (e.g. ST2, galectin 3, copeptin, adrenomedullin), there is no definite evidence to recommend them for clinical practice.”69
However, in a 2021 update, ESC (with special contribution from the Heart Failure Association) lists the key elements for HF and CMP diagnostic workups, and among the laboratory exams recommended under Table 26 for the “Initial diagnostic assessment in patients with suspected cardiomyopathy,” they include the use of ST2:
“Laboratory exams including cardiac and muscular enzymes, liver and renal function, haemoglobin, white blood cell count (including differential white blood cell count to detect eosinophilia), natriuretic peptides, thyroid function tests, iron status, and markers of systemic auto-immune disease (hsCRP, anti-nuclear antibodies, soluble IL-2 receptor).”70
Heart Failure Association of the European Society of Cardiology
The Heart Failure Association of the ESC published a position statement on Advanced Heart Failure which states: “Post-transplant patients should undergo a pre-defined regimen of graft biopsies, titration of immunosuppressive and other therapies, rejection monitoring, assessment for infections, transplant coronary artery disease and/or cardiac allograft vasculopathy, immunosuppression side effects, and other potential complications including neoplasia, and co-morbidities that require comprehensive treatment.” However, the guideline does not mention sST2 regarding prognosis of post-transplant patients.71
The Heart Failure Association of the ESC also released a consensus statement on HF diagnosis in the general community where they recommend the measurement of BNP or NT-proBNP as a key test in the assessment of patients with suspected signs and symptoms of heart failure. The association goes in to add that “there are currently no ESC guideline recommendations to support population-level screening for heart failure, however targeted screening is recommended in patients receiving cardiotoxic cancer treatments and there is increasing interest in prospectively testing the value of targeted screening strategies in high-risk populations such as those with diabetes and chronic kidney disease.”72
ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate Use Criteria for Coronary Revascularization in Patients with Acute Coronary Syndromes Guidelines
In 2016 The ACC, SCAI, Society of Thoracic Surgeons (STS), and American Association for Thoracic Surgery (AATS), along with key specialty and subspecialty societies created an Appropriate Use Task Force with the mission to revise the appropriate use criteria (AUC) for coronary revascularization. They have used clinical scenarios to mimic patient presentations seen in everyday clinical practice and included information on symptom status, presence of clinical instability or ongoing ischemic symptoms and other characteristics. They follow 2014 AHA/ACC recommendations for the use of cardiac biomarkers.4
American Society for Clinical Pathology (ASCP)
The ASCP recommends against testing CKMB or myoglobin to diagnose an acute MI. Instead, they recommend testing either troponin I or T. They also assert that both troponins are specific to cardiac injury and that there is much support for relying solely on troponin.73
National Institute for Health and Care Excellence (NICE)
National Institute for Health and Care Excellence recommends diagnosis of MI using the “detection of rise and/or fall of cardiac biomarkers values [preferably cardiac troponin (cTn)] with at least one value above the 99th percentile of the upper reference limit and at least one of the following:
- symptoms of ischaemia
- new or presumed new significant ST‑segment‑T wave (ST‑T) changes or new left bundle branch block (LBBB)
- development of pathological Q waves in the ECG
- imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
- identification of an intracoronary thrombus by angiography.”74
Currently, the 2018 NICE recommendations on chronic HF do not mention the usage of ST2 as a marker for diagnosing chronic HF. Instead, they recommend to “measure N-terminal pro-B-type natriuretic peptide (NT-proBNP) in people with suspected heart failure.”75
In 2020, NICE released recommendations on the use of high-sensitivity troponin tests to help rule-out NSTEMI earlier in those presenting to an ED with chest pain and suspected acute coronary syndrome. NICE recommends the use of the following assays: Access High-Sensitivity Troponin I Assay, ADVIA Centaur High-Sensitivity Cardiac Troponin‑I Assay, Alinity High Sensitive Troponin‑I assay, ARCHITECT STAT High Sensitive Troponin‑I assay, Atellica IM High-Sensitivity Cardiac Troponin I Assay, Dimension Vista High-Sensitivity Cardiac Troponin I Assay, Dimension EXL High-Sensitivity Cardiac Troponin I Assay, Elecsys Troponin T-high sensitive assay, Elecsys Troponin T-high sensitive STAT assay, VIDAS High sensitive Troponin I assay, and VITROS High-Sensitivity Troponin I Assay. NICE mentions that although the “TriageTrue test has the potential to be cost effective, its diagnostic accuracy when used on whole blood is uncertain.”76 Regarding use of these assays, NICE recommends using a threshold at or near the limit of detection, which varies depending on the assay used. If this sample is positive, it should not be used to rule in NSTEMI. If taking multiple samples, take a sample at initial assessment followed by a second sample taken 30 minutes to three hours after. Use 99th percentile thresholds or thresholds at or near the limit of detection of the assay.76
ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes
In 2025 the ACC/AHA/ACEP/NAEMSP/SCAI released guidelines for the management of patients with ACS. The committee states that cTn is the biomarker of choice for assessing patients for possible cardiac injury, with hs-cTn assays being preferred because the sensitivity and negative predictive values are greater. In addition, the time interval from chest pain onset to detection of hs-cTn is shorter with hs-cTn compared with conventional cTn assays, leading to more rapid “rule in” or “rule-out” of ischemia. cTn is central to the diagnosis of AMI. Given its high-sensitivity and specificity for myocardial injury, cTn (I or T) should be utilized to detect or exclude myocardial injury.77
The guidelines then go on to state that “higher cTn levels in patients with ACS are associated with an increased risk of death and MACE and similarly may be useful for helping to guide some treatment decisions. Several other serum or plasma biomarkers may provide additional prognostic information. In particular, natriuretic peptides (including B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide) may identify those patients with ACS at increased risk of death, HF, and recurrent MACE.”77
References
- Thygesen K, Alpert JS, White HD. Universal Definition of Myocardial Infarction. Circulation. 2007;116(22):2634-2653. doi:10.1161/circulationaha.107.187397
- Bayes-Genis A, Zhang Y, Ky B. ST2 and patient prognosis in chronic heart failure. The American journal of cardiology. Apr 02 2015;115(7 Suppl):64b-9b. doi:10.1016/j.amjcard.2015.01.043
- Reeder G, S., Awtry, E., Mahler, S., A. Initial evaluation and management of suspected acute coronary syndrome (myocardial infarction, unstable angina) in the emergency department. Updated May 30, 2025. https://www.uptodate.com/contents/initial-evaluation-and-management-of-suspected-acute-coronary-syndrome-myocardial-infarction-unstable-angina-in-the-emergency-department
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014;130(25):e344-e426. doi:10.1161/cir.0000000000000134
- WHO. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. 1979;doi:10.1161/01.CIR.59.3.607
- Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126(16):2020-2035. doi:10.1161/CIR.0b013e31826e1058
- Danese E, Montagnana M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Annals of translational medicine. May 2016;4(10):194. doi:10.21037/atm.2016.05.19
- Colucci W. Overview of the management of heart failure with reduced ejection fraction in adults. Updated March 4, 2024. https://www.uptodate.com/contents/overview-of-the-management-of-heart-failure-with-reduced-ejection-fraction-in-adults
- Colucci W, Dunlay S. Clinical manifestations and diagnosis of advanced heart failure. Updated November 11, 2024. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-advanced-heart-failure
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: Executive Summary. 2013-10-15 2013;doi:10.1161/CIR.0b013e31829e8807
- Colucci W. Prognosis of heart failure. Updated September 1, 2023. https://www.uptodate.com/contents/prognosis-of-heart-failure
- Marshall T, Williams J, Williams KM. Electrophoresis of serum isoenzymes and proteins following acute myocardial infarction. Journal of chromatography. Sep 13 1991;569(1-2):323-45. doi:10.1016/0378-4347(91)80236-6
- Jaffe AS, Morrow DA. Use of creatine kinase to detect myocardial injury. Updated September 29, 2023. https://www.uptodate.com/contents/use-of-creatine-kinase-to-detect-myocardial-injury
- Eggers KM, Oldgren J, Nordenskjold A, Lindahl B. Diagnostic value of serial measurement of cardiac markers in patients with chest pain: limited value of adding myoglobin to troponin I for exclusion of myocardial infarction. American heart journal. Oct 2004;148(4):574-81. doi:10.1016/j.ahj.2004.04.030
- Kavsak PA, MacRae AR, Newman AM, et al. Effects of contemporary troponin assay sensitivity on the utility of the early markers myoglobin and CKMB isoforms in evaluating patients with possible acute myocardial infarction. Clinica chimica acta; international journal of clinical chemistry. May 1 2007;380(1-2):213-6. doi:10.1016/j.cca.2007.01.001
- Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annual review of biochemistry. 1985;54:831-62. doi:10.1146/annurev.bi.54.070185.004151
- Penttila I, Penttila K, Rantanen T. Laboratory diagnosis of patients with acute chest pain. Clinical chemistry and laboratory medicine. Mar 2000;38(3):187-97. doi:10.1515/cclm.2000.027
- Dillon MC, Calbreath DF, Dixon AM, et al. Diagnostic problem in acute myocardial infarction: CK-MB in the absence of abnormally elevated total creatine kinase levels. Archives of internal medicine. Jan 1982;142(1):33-8. doi:10.1001/archinte.1982.00340140035009
- Heller GV, Blaustein AS, Wei JY. Implications of increased myocardial isoenzyme level in the presence of normal serum creatine kinase activity. The American journal of cardiology. Jan 1 1983;51(1):24-7. doi:10.1016/s0002-9149(83)80006-x
- Yusuf S, Collins R, Lin L, Sterry H, Pearson M, Sleight P. Significance of elevated MB isoenzyme with normal creatine kinase in acute myocardial infarction. The American journal of cardiology. Feb 1 1987;59(4):245-50. doi:10.1016/0002-9149(87)90793-4
- Engel G, Rockson SG. Feasibility and Reliability of Rapid Diagnosis of Myocardial Infarction. The American Journal of the Medical Sciences. 2020/02/01/ 2020;359(2):73-78. doi:10.1016/j.amjms.2019.12.012
- Greaser ML, Gergely J. Reconstitution of troponin activity from three protein components. The Journal of biological chemistry. Jul 10 1971;246(13):4226-33. doi:10.1016/S0021-9258(18)62075-7
- Bodor GS, Porterfield D, Voss EM, Smith S, Apple FS. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue. Clinical chemistry. Dec 1995;41(12 Pt 1):1710-5.
- Bodor GS, Survant L, Voss EM, Smith S, Porterfield D, Apple FS. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clinical chemistry. Mar 1997;43(3):476-84. doi:10.1093/clinchem/43.3.476
- Anderson PA, Malouf NN, Oakeley AE, Pagani ED, Allen PD. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circulation research. Nov 1991;69(5):1226-33. doi:10.1161/01.res.69.5.1226
- Saggin L, Gorza L, Ausoni S, Schiaffino S. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development (Cambridge, England). Oct 1990;110(2):547-54. doi:10.1242/dev.110.2.547
- McLaurin MD, Apple FS, Voss EM, Herzog CA, Sharkey SW. Cardiac troponin I, cardiac troponin T, and creatine kinase MB in dialysis patients without ischemic heart disease: evidence of cardiac troponin T expression in skeletal muscle. Clinical chemistry. Jun 1997;43(6 Pt 1):976-82. doi:10.1093/clinchem/43.6.976
- Neumann JT, Twerenbold R, Ojeda F, et al. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N Engl J Med. Jun 27 2019;380(26):2529-2540. doi:10.1056/NEJMoa1803377
- Anand A, Shah ASV, Beshiri A, Jaffe AS, Mills NL. Global Adoption of High-Sensitivity Cardiac Troponins and the Universal Definition of Myocardial Infarction. Clinical chemistry. Mar 2019;65(3):484-489. doi:10.1373/clinchem.2018.298059
- Boeddinghaus J, Nestelberger T, Koechlin L, et al. Early Diagnosis of Myocardial Infarction With Point-of-Care High-Sensitivity Cardiac Troponin I. Journal of the American College of Cardiology. 2020;75(10):1111-1124. doi:10.1016/j.jacc.2019.12.065
- Glatz JF, van der Vusse GJ. Cellular fatty acid-binding proteins: current concepts and future directions. Molecular and cellular biochemistry. Oct 15-Nov 8 1990;98(1-2):237-51. doi:10.1007/bf00231390
- Van Nieuwenhoven FA, Kleine AH, Wodzig WH, et al. Discrimination between myocardial and skeletal muscle injury by assessment of the plasma ratio of myoglobin over fatty acid-binding protein. Circulation. Nov 15 1995;92(10):2848-54. doi:10.1161/01.CIR.92.10.2848
- Seino Y, Ogata K, Takano T, et al. Use of a whole blood rapid panel test for heart-type fatty acid-binding protein in patients with acute chest pain: comparison with rapid troponin T and myoglobin tests. The American journal of medicine. Aug 15 2003;115(3):185-90. doi:10.1016/s0002-9343(03)00325-5
- Nguyen TN, Le PXM, Le TX, et al. The Value of Heart-Fatty Acid Binding Protein (H-FABP) in the Early Diagnostic of Patients with Acute Myocardial Infarction. Journal of the American College of Cardiology. 2020;75(11_Supplement_1):18-18. doi:10.1016/S0735-1097(20)30645-8
- Khan SQ, Dhillon OS, O’Brien RJ, et al. C-Terminal Provasopressin (Copeptin) as a Novel and Prognostic Marker in Acute Myocardial Infarction. Leicester Acute Myocardial Infarction Peptide (LAMP) Study. 2007;115(16):2103-2110. doi:10.1161/circulationaha.106.685503
- Maisel A, Mueller C, Neath S-X, et al. Copeptin Helps in the Early Detection of Patients With Acute Myocardial Infarction: Primary Results of the CHOPIN Trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction). Journal of the American College of Cardiology. 2013/07/09/ 2013;62(2):150-160. doi:10.1016/j.jacc.2013.04.011
- Hillinger P, Twerenbold R, Jaeger C, et al. Optimizing Early Rule-Out Strategies for Acute Myocardial Infarction: Utility of 1-Hour Copeptin. Clinical chemistry. 2015;61(12):1466-1474. doi:10.1373/clinchem.2015.242743
- Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. The American journal of cardiology. Apr 02 2015;115(7 Suppl):3b-7b. doi:10.1016/j.amjcard.2015.01.034
- Januzzi JL, Mebazaa A, Di Somma S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. The American journal of cardiology. Apr 02 2015;115(7 Suppl):26b-31b. doi:10.1016/j.amjcard.2015.01.037
- FDA. Substantial Equivalence Determination. https://www.accessdata.fda.gov/cdrh_docs/reviews/K111452.pdf
- Wilson S Colucci HHC. Natriuretic peptide measurement in heart failure. Updated March 11, 2024. https://www.uptodate.com/contents/natriuretic-peptide-measurement-in-heart-failure
- Roche. Roche CARDIAC Trop T Sensitive test (visual). https://diagnostics.roche.com/global/en/products/params/roche-cardiac-trop-t-sensitive-test-visual.html
- ResponseBio. Point of Care Cardiac. https://responsebio.com/acute-care-diagnostics/cardiovascular/
- Jeong JH, Seo YH, Ahn JY, et al. Performance of Copeptin for Early Diagnosis of Acute Myocardial Infarction in an Emergency Department Setting. Ann Lab Med. Jan 2020;40(1):7-14. doi:10.3343/alm.2020.40.1.7
- Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circulation Heart failure. Mar 2011;4(2):180-7. doi:10.1161/circheartfailure.110.958223
- Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. Sep 25 2012;126(13):1596-604. doi:10.1161/circulationaha.112.129437
- Wijk SS-v, Maeder MT, Nietlispach F, et al. Long-Term Results of Intensified, N-Terminal-Pro-B-Type Natriuretic Peptide–Guided Versus Symptom-Guided Treatment in Elderly Patients With Heart Failure. Circulation: Heart Failure. 2014;7(1):131-139. doi:10.1161/CIRCHEARTFAILURE.113.000527
- Wang Y, Tan X, Gao H, et al. Magnitude of Soluble ST2 as a Novel Biomarker for Acute Aortic Dissection. Circulation. Jan 16 2018;137(3):259-269. doi:10.1161/circulationaha.117.030469
- Januzzi JL, Horne BD, Moore SA, et al. Interleukin receptor family member ST2 concentrations in patients following heart transplantation. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. May 2013;18(3):250-6. doi:10.3109/1354750x.2013.773081
- Boman K, Thormark Frost F, Bergman AR, Olofsson M. NTproBNP and ST2 as predictors for all-cause and cardiovascular mortality in elderly patients with symptoms suggestive for heart failure. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. May - Jun 2018;23(4):373-379. doi:10.1080/1354750x.2018.1431692
- Dimitropoulos S, Mystakidi VC, Oikonomou E, et al. Association of Soluble Suppression of Tumorigenesis-2 (ST2) with Endothelial Function in Patients with Ischemic Heart Failure. Int J Mol Sci. Dec 9 2020;21(24)doi:10.3390/ijms21249385
- Hou ZW, Yu HB, Liang YC, et al. Circulating Soluble ST2 Predicts All-Cause Mortality in Severe Heart Failure Patients with an Implantable Cardioverter Defibrillator. Cardiol Res Pract. 2020;2020:4375651. doi:10.1155/2020/4375651
- Pop-Busui R, Januzzi JL, Bruemmer D, et al. Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022;45(7):1670-1690. doi:10.2337/dci22-0014
- Jarolim P, White WB, Cannon CP, Gao Q, Morrow DA. Serial Measurement of Natriuretic Peptides and Cardiovascular Outcomes in Patients With Type 2 Diabetes in the EXAMINE Trial. Diabetes Care. Jul 2018;41(7):1510-1515. doi:10.2337/dc18-0109
- Committee ADAPP. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2025. Diabetes Care. 2024;48(Supplement_1):S207-S238. doi:10.2337/dc25-S010
- Jaffe AS, Chaitman BR, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). European Heart Journal. 2018;40(3):237-269. doi:10.1093/eurheartj/ehy462
- Tamis-Holland Jacqueline E, Jneid H, Reynolds Harmony R, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019/04/30 2019;139(18):e891-e908. doi:10.1161/CIR.0000000000000670
- Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. Oct 22 2013;62(17):1563-70. doi:10.1016/j.jacc.2013.08.720
- O’Connor Robert E, Al Ali Abdulaziz S, Brady William J, et al. Part 9: Acute Coronary Syndromes. Circulation. 2015/11/03 2015;132(18_suppl_2):S483-S500. doi:10.1161/CIR.0000000000000263
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2017;70(6):776-803. doi:10.1016/j.jacc.2017.04.025
- Heidenreich PA, Bozkurt, B., Aguilar, D., Allen, L. A., Byun, J. J., Colvin, M. M., Deswal, A., Drazner, M. H., Dunlay, S. M., Evers, L. R., Fang, J. C., Fedson, S. E., Fonarow, G. C., Hayek, S. S., Hernandez, A. F., Khazanie, P., Kittleson, M. M., Lee, C. S., Link, M. S., & Milano, C. A. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. Journal of the American College of Cardiology. 2022;doi:10.1016/j.jacc.2021.12.012
- Chow SL, Maisel AS, Anand I, et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017/05/30 2017;135(22):e1054-e1091. doi:10.1161/CIR.0000000000000490
- Cresci S, Pereira Naveen L, Ahmad F, et al. Heart Failure in the Era of Precision Medicine: A Scientific Statement From the American Heart Association. Circulation: Genomic and Precision Medicine. 2019/10/01 2019;12(10):e000058. doi:10.1161/HCG.0000000000000058
- Gencer B, Brotons C, Mueller C, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). European Heart Journal. 2016;37(3):267-315. doi:10.1093/eurheartj/ehv320
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal. 2019;41(3):407-477. doi:10.1093/eurheartj/ehz425
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. Apr 7 2021;42(14):1289-1367. doi:10.1093/eurheartj/ehaa575
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal. 2023;44(38):3720-3826. doi:10.1093/eurheartj/ehad191
- Vrints C, Andreotti F, Koskinas KC, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). European Heart Journal. 2024;45(36):3415-3537. doi:10.1093/eurheartj/ehae177
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal. 2016;37(27):2129-2200. doi:10.1093/eurheartj/ehw128
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. Sep 21 2021;42(36):3599-3726. doi:10.1093/eurheartj/ehab368
- Crespo-Leiro MG, Metra M, Lund LH, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. European journal of heart failure. May 27 2018;doi:10.1002/ejhf.1236
- Docherty KF, Lam CSP, Rakisheva A, et al. Heart failure diagnosis in the general community – Who, how and when? A clinical consensus statement of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). European journal of heart failure. 2023;25(8):1185-1198. doi:10.1002/ejhf.2946
- ASCP. Don’t test for myoglobin or CK-MB in the diagnosis of acute myocardial infarction (AMI). Instead, use troponin I or T. https://www.aafp.org/pubs/afp/collections/choosing-wisely/246.html
- NICE. Chest pain of recent onset: assessment and diagnosis. Updated November 30, 2016. https://www.nice.org.uk/guidance/cg95/chapter/Recommendations
- NICE. Chronic heart failure in adults: diagnosis and management. Updated September 12, 2018. https://www.nice.org.uk/guidance/ng106
- NICE. High-sensitivity troponin tests for the early rule out of NSTEMI. Updated August 26, 2020. https://www.nice.org.uk/guidance/dg40/chapter/1-Recommendations
- Rao SV, O’Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients With Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 0(0)doi:10.1161/CIR.0000000000001309
Coding Section
| Code | Number | Description |
| CPT | 82550 | Creatine kinase (CK), (CPK); total |
| 82552 | Creatine kinase (CK), (CPK); isoenzymes |
|
| 82553 | Creatine kinase (CK), (CPK); MB fraction only | |
| 82554 | Creatine kinase (CK), (CPK); isoforms | |
| 82725 | Fatty acids, nonesterified |
|
| 83006 | Growth stimulation expressed gene 2 (ST2, Interleukin 1 receptor like-1) | |
| 83615 | Lactate dehydrogenase (LD), (LDH); |
|
| 83625 | Lactate dehydrogenase (LD), (LDH); isoenzymes, separation and quantitation | |
| 83874 | Myoglobin | |
| 83880 | Natriuretic peptide | |
| 84450 | Transferase; aspartate amino (AST) (SGOT | |
| 84484 | Troponin, quantitative | |
| 84512 | Troponin, qualitative | |
| 84588 | Vasopressin (antidiuretic hormone, ADH) | |
| 84999 | Unlisted chemistry code | |
| 86140 | C-reactive protein | |
| 86141 | C-reactive protein; high sensitivity (hsCRP) | |
| ICD-10 Diagnoses | F41.0 | Panic disorder [episodic paroxysmal anxiety], Panic attack, Panic state |
| F41.9 | Anxiety disorder, unspecified | |
| K230 | Indigestion | |
| M25.511 | Pain in right shoulder | |
| M25.512 | Pain in left shoulder | |
| M25.519 | Pain in unspecified shoulder(discomfort) | |
| M54.5, M54.6, M54.9 | Lower Back Pain, Pain in Thoracic spine, Dorsalgia (Back pain NOS) | |
| M79.601, M79.621 | Pain in right arm | |
| M79.602, M79.622 | Pain in left arm | |
| M79.603, M79.629 | Pain in limb, arm | |
| R00.2 | Palpitations |
|
| R06.02 | Shortness of breath | |
| R07.1 | Chest pain on breathing | |
| R07.89 | Other chest pain | |
| R07.9 | Chest pain, unspecified | |
| R10.0 | Acute Abdominal pain | |
| R10.10-R10.13 | Pain localized to upper abdomen | |
| R10.30-R10.33 | Pain localized to other parts of lower abdomen | |
| R10.81 | Abdominal tenderness | |
| R10.84 | Generalized abdominal pain | |
| R10.9 | Unspecified abdominal pain | |
| R11.0 | Nausea | |
| R11.10 | Vomiting, unspecified | |
| R11.2 | Nausea with vomiting, unspecified | |
| R12 | Heartburn | |
| R23.1 | Clammy skin/Pallor | |
| R42 | Dizziness and giddiness/Light-headedness | |
| R53.1, R53.8 | Fatigue | |
| R61 | Sweating | |
| R68.83 | Chills (without fever)-Cold Sweats | |
| R68.84 | Jaw pain |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 07/23/2025 | Annual review, updating description, changing the order of criteria, table of terminology, rationale, and references. Also adding CPT 86141. |
| 10/07/2024 | Annual review, no change to policy intent. Updating rationale and references. |
| 11/02/2023 | Interim review, review month changed to October. Entire policy updated for clarity and consistency. Adding verbiage regarding BNP testing. CAM 049 will archive when this policy is published. |
| 07/25/2023 | Annual review, updating title and policy as the information in CAM 295 is being merged into this policy. Also updating description, note table of terminology, rationale and references. Adding CPT 83006. |
| 07/20/2022 | Annual review, no change to policy intent. |
| 07/13/2021 |
Annual review, no change to policy intent. Updating background/description, rationale and references. |
| 07/06/2020 |
Annual review, no change to policy intent. Updating description, rationale and references. |
| 07/12/2019 |
Annual review, no change to policy intent. |
| 04/17/2019 |
Updating next review date. No other changes. |
| 05/15/2018 |
Updating effective date to reflect 8/13/2018. No other changes. |
| 05/10/2018 |
New Policy |