Low-Dose CT for Lung Cancer Screening - CAM 391
GENERAL INFORMATION
- It is an expectation that all patients receive care/services from a licensed clinician. All appropriate supporting documentation, including recent pertinent office visit notes, laboratory data, and results of any special testing must be provided. If applicable: All prior relevant imaging results and the reason that alternative imaging cannot be performed must be included in the documentation submitted.
- Where a specific clinical indication is not directly addressed in this guideline, medical necessity determination will be made based on widely accepted standard of care criteria. These criteria are supported by evidence-based or peer-reviewed sources such as medical literature, societal guidelines and state/national recommendations.
- The guideline criteria in the following sections were developed utilizing evidence-based and peer-reviewed resources from medical publications and societal organization guidelines as well as from widely accepted standard of care, best practice recommendations.
Purpose
Low Dose Computed Tomography (LDCT) generates images of the lungs (chest) and is used to screen for and detect lung cancer in high-risk patients and/or patients with a history of lung cancer. This study uses low doses of radiation (100-120 kVp and 40-60 mAs) and is primarily used to evaluate the lung parenchyma. When evaluation of structures such as lymph nodes or the mediastinum is needed, a standard dose CT with IV contrast may be more appropriate.(1)
Policy
IINDICATIONS
For Annual Screening
The use of low-dose, non-contrast spiral (helical) multi-detector CT imaging as a screening technique for lung cancer is considered medically necessary ONLY when used to screen for lung cancer for certain high-risk, asymptomatic individuals (i.e., no acute lung-related symptoms, when ALL of the following criteria are met)
NOTE: Screening should be discontinued once a person develops a health problem that limits the willingness or ability to have curative intent treatment.(2)
Group 1 - High Risk for Lung Cancer(3,4)
- Individual is between 50-80 years of age*; AND
- There is at least a 20 pack-year history of cigarette** smoking
*May approve for individuals over the age limit if the individual is a candidate for and willing to undergo curative treatment upon diagnosis.
** Only personal cigarette smoking history as above places an individual at high risk; secondhand smoke exposure and other forms of smoking (such as pipe, cigar, marijuana, vaping) do NOT factor into current recommendations for LDCT Screening.
Group 2 - Personal History of Lung Cancer(1)
Low Dose CT is indicated for surveillance of non-small cell lung cancer as follows:
- Annually starting 3 years after the end of treatment if stage I-II and no history of radiation
- Annually starting 6 years after end of treatment if EITHER stage I-II with history of radiation OR stage III or IV
NOTE: While on treatment, and for the first 2-3 years after completion of treatment, surveillance is with Chest CT rather than LDCT. When radiation was used for treatment, chest CT is needed for longer (5 years) before LDCT is appropriate. LDCT is not used for surveillance of small cell lung cancer (5) (see Evolent Clinical Guideline 2018 for Chest CT)
Follow up of nodule on initial LDCT(3)
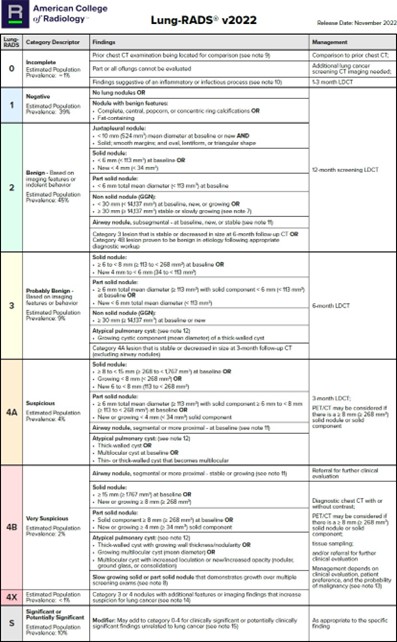
- Table 1 below shows the follow-up interval at which LDCT is indicated for follow up of nodules(2)
- If multiple nodules, the largest and most concerning is used for decision
Table 1: Lung-RADS®(2)
*This table is reproduced without alteration or edit in accordance with provisions in a Creative Commons License. The full document and license information can be found here: Lung RADS | American College of Radiology (acr.org)
Rationale
SUMMARY OF EVIDENCE
ACR Lung-RADS v2022: Assessment Categories and Management Recommendations(2)
Study Design: This article provides an update to the Lung CT Screening Reporting and Data System (Lung-RADS) developed by the American College of Radiology (ACR). The updates are based on new scientific evidence, expert consensus, and systematic reviews of the literature.
Target Population: The target population includes individuals undergoing lung cancer screening with low-dose CT (LDCT), particularly those at high risk for lung cancer.
Key Factors: Introduces new criteria for atypical pulmonary cysts, juxtapleural nodules, airway-centered nodules, and potentially infectious findings. Provides updated management recommendations for various nodule types and conditions. Clarifies the definition of nodule growth and introduces stepped management for nodules that are stable or decreasing in size.
Screening for lung cancer: 2023 guideline update from the American Cancer Society(4)
Study Design: This guideline update is based on a systematic review of the literature, epidemiologic and modeling analyses, and expert consensus. The American Cancer Society (ACS) Guideline Development Group (GDG) utilized various sources, including the US Preventive Services Task Force 2021 recommendation update, Cancer Intervention and Surveillance Modeling Network-validated lung cancer models, and disease burden data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program.
Target Population: The guideline targets adults at high risk for lung cancer due to a history of smoking. Specifically, it recommends annual lung cancer screening (LCS) with low-dose computed tomography (LDCT) for asymptomatic individuals aged 50-80 years who currently smoke or formerly smoked and have a ≥20 pack-year smoking history.
Key Factors: Individuals aged 50-80 years with a ≥20 pack-year smoking history, regardless of years since quitting smoking (YSQ). Annual LCS with LDCT for eligible individuals. Emphasizes the importance of shared decision-making between patients and healthcare providers. Individuals who currently smoke should receive counseling to quit and be connected to cessation resources. Individuals with comorbid conditions that substantially limit life expectancy should not be screened.
ANALYSIS OF EVIDENCE
Analysis(2,4):
Both articles provide strong evidence supporting the use of LDCT for lung cancer screening in high-risk individuals. They agree on the significant mortality reduction achieved through regular screening and the importance of identifying eligible individuals based on age and smoking history. However, they differ in their recommendations regarding the YSQ criterion, management of findings, and the use of volumetric analysis. These differences highlight the need for ongoing research and individualized patient care to optimize lung cancer screening outcomes.
Shared Findings
Both articles emphasize the importance of LDCT in reducing lung cancer mortality among high-risk individuals. They highlight the significant reduction in lung cancer-specific mortality demonstrated by major trials such as the National Lung Screening Trial (NLST) and the Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON).
- Mortality Reduction: Both articles agree that LDCT screening leads to a substantial reduction in lung cancer mortality. The NLST reported a 20% reduction in lung cancer-specific mortality, while the NELSON trial reported reductions of up to 26% and 39% in lung cancer-specific mortality.
- Eligibility Criteria: Both guidelines recommend LDCT screening for individuals aged 50-80 years with a significant smoking history (≥20 pack-years). They emphasize the importance of identifying high-risk individuals for screening.
- Screening Intervals: Both articles support annual screening with LDCT for eligible individuals. They stress the importance of regular screening to detect lung cancer at an early, more treatable stage.
References
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer Version 3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Christensen J, Prosper AE, Wu CC, et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Journal of the American College of Radiology. 2024;21(3):473-488. doi:10.1016/j.jacr.2023.09.009
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Lung Cancer Screening Version 1.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Wolf AMD, Oeffinger KC, Shih TY, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2024;74(1):50-81. doi:10.3322/caac.21811
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Cell Lung Cancer Version 4.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org
Coding Section
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2023 Forward
| 01/13/2026 | Annual review, no change to policy intent. Updating policy language for clarity and consistency, background, and rationale. Adding statement to general information. |
| 09/01/2025 | Annual review, no change to policy intent. |
| 09/24/2024 | Annual review, no change to policy intent. Updating references and Lung Rads table, policy reformatted for clarity and consistency. |
| 10/24/2023 |
New Policy |