Pancreatic Enzyme Testing for Acute Pancreatitis - CAM 198
Description
Pancreatitis is an inflammation of pancreatic tissue and can be either acute or chronic. Pancreatic enzymes, including amylase, lipase, and trypsinogen, can be used to monitor the relative health of the pancreatic tissue. Damage to the pancreatic tissue, including pancreatitis, can result in elevated pancreatic enzyme concentrations whereas depressed enzyme levels are associated with exocrine pancreatic insufficiency.1,2
Regulatory Status
The FDA has approved multiple tests for human serum total as well as for pancreatic. FDA Device database accessed on 5/30/2018 yielded 141 records for test.
Lipase
The FDA has approved multiple tests for human serum lipase. FDA Device database accessed on May 30, 2018, yielded 51 records for lipase test.
Trypsinogen/Trypsin/TAP
Trypsin immunostaining, trypsinogen-2 dipstick, and TAP serum tests are considered laboratory developed tests (LDT); developed, validated and performed by individual laboratories.
LDTs are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88).
As an LDT, the U.S. Food and Drug Administration has not approved or cleared this test; however, FDA clearance or approval is not currently required for clinical use.
CRP
The FDA has approved multiple tests for human CRP, including assays for conventional CRP, high sensitivity CRP (hsCRP), and cardiac CRP (cCRP). On Sept. 22, 2005, the FDA issued guidelines concerning the assessment of CRP (FDA, 2005).
Procalcitonin
On April 18, 2017, the FDA approved the Diazyme Procalcitonin PCT Assay, Diazyme Procalcitonin Calibrator Set, and Diazyme Procalcitonin Control Set as substantially equivalent and has received FDA 510K clearance for marketing.
IL-6/IL-8
IL-6 and IL-8 are ELISA-based tests and are considered laboratory developed tests (LDT); developed, validated and performed by individual laboratories. IL-6 and IL-8 can also be components of a cytokine panel test, which is also an LDT.
LDTs are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA’88).
As an LDT, the U.S. Food and Drug Administration has not approved or cleared this test; however, FDA clearance or approval is not currently required for clinical use.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- For individuals presenting with signs and symptoms of acute pancreatitis (see Note 1), measurement of serum lipase is considered MEDICALLY NECESSARY.
- Measurement of serum lipase is considered NOT MEDICALLY NECESSARY in any of the following situations:
- For individuals with an established diagnosis of acute or chronic pancreatitis.
- More than once per visit.
- For asymptomatic individuals during a general exam without abnormal findings.
- When ordered for anything other than analysis of pancreatic cyst fluid, measurement of serum is considered NOT MEDICALLY NECESSARY.
- For the diagnosis, assessment, prognosis, and/or determination of severity of acute pancreatitis, measurement of serum or urine trypsin/trypsinogen/TAP (trypsinogen activation peptide) is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of an individual’s illness.
- For the diagnosis, assessment, prognosis, and/or determination of severity of acute pancreatitis, measurement of the following biomarkers is considered NOT MEDICALLY NECESSARY:
- C-Reactive Protein (CRP)
- Interleukin-6 (IL-6)
- Interleukin-8 (IL-8)
- Procalcitonin
- For individuals presenting with signs and symptoms of acute pancreatitis (see Note 1), measurement of urinary concentration for the initial diagnosis of acute pancreatitis is considered NOT MEDICALLY NECESSARY.
- For all other situations or conditions not described above, measurement of serum lipase is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: Signs and symptoms of acute pancreatitis:3,4
- Mild to severe epigastric pain that begins slowly or suddenly (may spread to the back in some patients)
- Nausea
- Vomiting
- Tender to palpitation of epigastrium
- Abdominal distention
- Hypoactive bowel sounds
- Fever
- Rapid pulse
- Tachypnea
- Hypoxemia
- Hypotension
- Anorexia
- Diarrhea
- Cullen sign
- Grey Turner sign
Table of Terminology
| Term |
Definition |
| AACC |
American Association for Clinical Chemistry |
| ABIM |
American Board of Internal Medicine |
| ACCR |
Amylase-to-creatinine clearance ratio |
| ACG |
American College of Gastroenterology |
| AED |
Academy For Eating Disorders |
| AGA |
American Gastroenterological Association |
| AP |
Acute pancreatitis |
| APA |
American Pancreatic Association |
| APA |
American Psychiatric Association |
| APACHE-II |
Acute Physiology and Chronic Health Evaluation |
| ASCP |
American Society for Clinical Pathology |
| AUC |
Area under the curve |
| BISAP |
Bedside index for severity in acute pancreatitis |
| BUN |
Blood urea nitrogen |
| CADTH |
Canadian Agency for Drugs and Technologies in Health |
| cCRP |
Cardiac C-reactive protein |
| CECT |
Contrast-enhanced computed tomography |
| CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CMS |
Centers for Medicare & Medicaid Services |
| CP |
Chronic pancreatitis |
| CPEC |
Clinical Practice and Economics Committee |
| CRP |
C-reactive protein |
| CT |
Computed axial tomography |
| CTSI |
Computed axial tomography severity index |
| ED |
Eating disorder |
| ELISA |
Enzyme-linked immunoassay |
| EPI |
Exocrine pancreatic insufficiency |
| ERCP |
Endoscopic retrograde cholangiopancreatography |
| EUS |
Endoscopic ultrasonography |
| FDA |
Food and Drug Administration |
| GRADE |
Grading of recommendations assessment, development, and evaluation |
| HIV |
Human immunodeficiency virus |
| HMGB1 |
High Mobility Group Box 1 |
| hsCRP |
High sensitivity C-reactive protein |
| HSROC |
Hierarchical summary receiver operating characteristics curve |
| IAP |
International Association of Pancreatology |
| IL-6 |
Interleukin-6 |
| IL-8 |
Interleukin-8 |
| LCD |
Local Coverage Determination |
| LDH |
Lactate dehydrogenase |
| LDT |
Laboratory-developed test |
| MODS |
Multiorgan dysfunction syndrome |
| MRCP |
Magnetic resonance cholangiopancreatography |
| MRI |
Magnetic resonance imaging |
| NASPGHAN |
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee |
| NCDs |
National Coverage Determinations |
| PBMC |
Peripheral blood mononuclear cell |
| PCT |
Procalcitonin |
| PICU |
Pediatric intensive care unit |
| POC |
Point of care |
| RIA |
Radioimmunoassay |
| SIRS |
Systemic inflammatory response syndrome |
| s-isoform |
Salivary glands |
| SPINK1 |
Serine protease inhibitor Kazal type 1 |
| TAP |
Trypsinogen activation peptide |
| ULN |
Upper limit of normal |
| URL |
Upper limit of reference interval |
| UTDT |
Urine trypsinogen dipstick test |
Rationale
Acute Pancreatitis
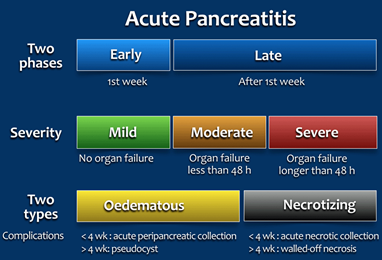
Acute pancreatitis (AP) is inflammation of the pancreatic tissue that can range in clinical manifestations. In approximately 80% of individuals, AP clears up completely or shows significant improvement within one to two weeks. However, it can sometimes lead to serious complications and as such, is often treated in a hospital.5 Due to the lack of consensus in diagnosing, characterizing, and treating AP, an international group of researchers and practitioners convened in Atlanta in 1992 to write a clinically based classification system for AP, which is now commonly referred to as the Atlanta convention or Atlanta classification system.6 The Atlanta classification system was revised in 2012.1 For the diagnosis of AP, two of the three following criteria must be present: “(1) abdominal pain consistent with acute pancreatitis (acute onset of a persistent, severe, epigastric pain often radiating to the back); (2) serum lipase activity (or amylase activity) at least three times greater than the upper limit of normal; and (3) characteristic findings of acute pancreatitis on contrast-enhanced computed tomography (CECT) and less commonly magnetic resonance imaging (MRI) or transabdominal ultrasonography” (italics emphasized by the manuscript’s authors).1 This two-of-three criterion is recommended for diagnostic use by several professional societies.7-9 AP can be characterized by two temporal phases, early or late, with degrees of severity ranging from mild (no organ failure) to moderate (organ failure less than 48 hours) to severe (persistent organ failure has occurred for more than 48 hours). There are two subclasses of AP: edematous AP and necrotizing AP. Edematous AP is due to inflammatory edema with relative homogeneity. Necrotizing AP displays necrosis of pancreatic and/or peripancreatic tissues.1 The figure below from Bollen, et al. (2015) outlines the revised Atlanta classification system of AP:
Chronic Pancreatitis
Chronic pancreatitis (CP) is an inflammation of the pancreatic tissue. The two hallmarks of CP are severe abdominal pain and pancreatic insufficiency.11 Alcohol-induced chronic pancreatitis (or alcohol pancreatitis) accounts for 40-70% of all cases of CP.12
The endocrine system is comprised of several glands which secrete hormones directly into the bloodstream to regulate many different bodily functions. The exocrine system is comprised of glands which secrete products through ducts, rather than directly into the bloodstream. CP affects both the endocrine and exocrine functions of the pancreas. Fibrogenesis occurs within the pancreatic tissue due to activation of pancreatic stellate cells by toxins (for example, those from chronic alcohol consumption) or cytokines from necroinflammation. Measuring the serum levels of amylase, lipase, and/or trypsinogen is not helpful in diagnosing CP since not every CP patient experiences acute episodes, the relative serum concentrations may be either decreased or unaffected, and the sensitivities of the tests are not enough to distinguish reduced enzyme levels.13 The best method to diagnose CP is to histologically analyze a pancreatic biopsy, but this invasive procedure is not always the most practical so “contrast-enhanced computed tomography is the best imaging modality for diagnosis. Computed tomography may be inconclusive in early stages of the disease, so other modalities such as magnetic resonance imaging, magnetic resonance cholangiopancreatography, or endoscopic ultrasonography with or without biopsy may be used.”14 Previously, ERCP was commonly used to diagnose CP, but the procedure can cause post-ERCP pancreatitis. Genetic factors are also implicated in CP, especially those related to trypsin activity, the serine protease inhibitor SPINK1, and cystic fibrosis.13,15,16
Amylase
Amylase is an enzyme produced predominantly in the salivary glands (s-isoform) and the pancreas (p-isoform or p-isoamylase) and is responsible for the digestion of polysaccharides, cleaving at the internal 1→4 alpha linkage. Up to 60% of the total serum amylase can be of the s-isoform. The concentration of total serum amylase as well as the pancreatic isoenzyme increase following pancreatic injury or inflammation.17,18 Even though the serum concentration of the pancreatic diagnostic enzymes, including amylase, lipase, elastase, and immunoreactive trypsin, all increase within 24 hours of onset of symptomology, amylase is the first pancreatic enzyme to return to normal levels so the timing of testing is of considerable importance for diagnostic value.17,19,20 The half-life of amylase is 12 hours since it is excreted by the kidneys, so its clinical value decreases considerably after initial onset of AP. The etiology of the condition can also affect the relative serum amylase concentration. In up to 50% of AP instances due to hypertriglyceridemia (high blood levels of triglycerides), the serum amylase concentration falls into the normal range, and normal concentrations of amylase has been reported in cases of alcohol-induced AP;17,21 in fact, one study shows that 58% of the cases of normoamylasemic AP was associated with alcohol use.22 Elevated serum amylase concentrations also can occur in conditions other than AP, including hyperamylasemia (excess amylase in the blood) due to drug exposure,23,24 bulimia nervosa,25 leptospirosis,26 and microamylasemia.18 Serum amylase levels are often significantly elevated in individuals with bulimia nervosa due to recurrent binge eating episodes.25 Macroamylasemia is a condition where the amylase concentration increases due to the formation of macroamylases, complexes of amylase with immunoglobulins and/or polysaccharides. Macroamylasemia is associated with other disease pathologies, “including celiac disease, HIV infection, lymphoma, ulcerative colitis, rheumatoid arthritis, and monoclonal gammopathy.” Suspected macroamylasemia in instances of isolated amylase elevation can be confirmed by measuring the amylase-to-creatinine clearance ratio (ACCR) since macroamylase complexes are too large to be adequately filtered. Normal values range from three to four percent with values of less than one percent supporting the diagnosis of macroamylasemia. ACCR itself is not a good indicator of AP since low ACCR is also exhibited in diabetic ketoacidosis and severe burns.18 Hyperamylasemia is also seen in other extrapancreatic conditions, such as appendicitis, salivary disease, gynecologic disease, extra-pancreatic tumors, and gastrointestinal disease.18,27 Gullo’s Syndrome (or benign pancreatic hyperenzymemia) is a rare condition that also exhibits high serum concentrations of pancreatic enzymes without showing other signs of pancreatitis.28 No correlation has been found between the concentration of serum amylase and the severity or prognosis of AP.29
Urinary amylase and peritoneal amylase concentrations can also be measured. Rompianesi, et al. (2017) reviewed the use of urinary amylase and trypsinogen as compared to serum amylase and serum lipase testing. The authors note that “with regard to urinary amylase, there is no clear-cut level beyond which someone with abdominal pain is considered to have acute pancreatitis.” Three studies regarding urinary amylase were reviewed —each with 134-218 participants—and used the hierarchical summary receiver operating characteristics curve (HSROC) analysis to compare the accuracy of the studies. Results showed that “the models did not converge” and the authors concluded that “we were therefore unable to formally compare the diagnostic performance of the different tests.”30
A study investigated the use of peritoneal amylase concentrations for diagnostic measures and discovered that patients with intra-abdominal peritonitis had a mean peritoneal amylase concentration of 816 U/L (142-1746 U/L range), patients with pancreatitis had a mean concentration of 550 U/L (100-1140 U/L range), and patients with other “typical infectious peritonitis” had a mean concentration of 11.1 U/L (0-90 U/L range). Conclusions state “that peritoneal fluid amylase levels were helpful in the differential diagnosis of peritonitis in these patients” and that levels >100 U/L “differentiated those patients with other intra-abdominal causes of peritonitis from those with typical infectious peritonitis.”31 The authors do not state if intraperitoneal amylase is specifically useful in diagnosing AP.
Liu, et al. (2021) conducted a retrospective cohort study to evaluate whether serum amylase and lipase could serve as a biomarker to predict pancreatic injury in 79 critically ill children who died of different causes. Through autopsy investigation, the subjects were divided into pancreatic injury group and non-pancreatic injury group. Forty-one patients (51.9%) exhibited pathological changes of pancreatic injury. Levels of lactate, erythrocyte sedimentation rate, alanine transaminase, aspartate transaminase, and troponin-I in the pancreatic injury group were significantly higher than that in the noninjury group. "Multivariable logistic regression analysis showed that serum amylase, serum lipase, and septic shock were significantly associated with the occurrence rate of pancreatic injury." Therefore, the authors conclude that "serum amylase and lipase could serve as independent biomarkers to predict pancreatic injury in critically ill children.”32
In a prospective case control study, Judal, et al. (2022) investigated urinary amylase levels for diagnosis of acute pancreatitis. One major challenge with measurement of serum amylase is its short half-life which returns to normal levels within a short period of time. This study enrolled 100 patients (50 healthy and 50 with acute pancreatitis) who were measured for serum amylase, serum lipase, and urinary amylase. There was a statistically significant increase in the serum amylase, lipase, and urinary amylase mean values of patients with AP. "Serum amylase had the highest sensitivity (100%) and serum lipase had the highest specificity (96.53%). The sensitivity and specificity of urinary amylase was found to be 97.25% and 91.47% respectively."33 The authors conclude that urinary amylase is a convenient and sensitive test for diagnosis.
Ryholt, et al. (2024) conducted a retrospective study with data collected throughout 2020 to “assess the utilization of appropriate laboratory testing related to the diagnosis of acute pancreatitis.” The authors were particularly interested in the overuse of amylase testing or amylase and lipase testing together when lipase testing alone would have been sufficient for AP diagnosis. Overall, 2567 (9.3%) of all amylase and lipase tests were determined to be unnecessary, an estimated $128,350 in total cost savings if eliminated. Of the unnecessary tests, 1881 (73.2%) were amylase tests and 686 (26.7%) were lipases tests. The authors also note that “an analysis of test-ordering behavior by providers revealed that 81.5% of all unnecessary tests were ordered by MDs.” The authors conclude that the “study demonstrated that amylase and lipase tests have been overutilized in the diagnosis of acute pancreatitis.”34
Mogekar, et al. (2024) studied the effectiveness of urinary amylase in diagnosing AP. The authors compared urinary amylase to serum amylase, with a focus on sensitivity and prolonged detection capabilities. The study included 60 patients suspected of AP with no significant comorbidities. Serum amylase and urinary amylase were measured in all patients. “The median serum amylase level was 311 U/L, while urinary amylase levels averaged 501 U/L.” The authors concluded that “elevated urinary amylase levels, which rise within 24 hours of symptom onset and can remain elevated for several days, provide a sensitive indicator of acute pancreatitis, especially in cases with late presentation.”35
Lipase (Pancreatic Lipase or Pancreatic Triacylglycerol Lipase)
Pancreatic lipase or triacylglycerol lipase (herein referred to as “lipase”) is an enzyme responsible for hydrolyzing triglycerides to aid in the digestion of fats. Like amylase, lipase concentration increases shortly after pancreatic injury (within three to six hours). However, contrary to amylase, serum lipase concentrations remain elevated for one to two weeks after initial onset of AP since lipase can be reabsorbed by the kidney tubules.29 Moreover, the pancreatic lipase concentration is 100-fold higher than the concentration of other forms of lipases found in other tissues such as the duodenum and stomach.17 Both the sensitivity and the specificity of lipase in laboratory testing of AP are higher than that of amylase.19 A study by Coffey, et al. (2013) found “an odds ratio of 7.1 (95% confidence interval 2.5-20.5; P<0.001) for developing severe AP” in patients ages 18 or younger when the serum lipase concentration is at least 7-fold higher than upper limit of normal. However, in general, elevated serum lipase concentration is not used to determine the severity or prognosis of AP.37 Hyperlipasemia can also occur in other conditions such as Gullo’s Syndrome.28 The use of lipase to determine etiology of AP is of debate. A study by Levy, et al. (2005) reports that lipase alone cannot be used to determine biliary cause of AP whereas other studies have indicated that a ratio of lipase-to-amylase concentrations ranging from 2:1 to more than 5:1 can be indicative of alcohol-induced AP.37,39-41
The review by Ismail and Bhayana (2017) included a summary table (Table 1 below) comparing various studies concerning the use of amylase and lipase for diagnosis of AP as well as a table (Table 2 below) comparing the cost implication of the elimination of double-testing for AP.
Table 1: Summary of numerous studies comparing lipase against amylase (URL – Upper Limit of Reference interval, AP – Acute Pancreatitis).
| Design and reference |
Participant (patients with abdominal pain/AP) |
Threshold |
Results |
Conclusion |
|
| Serum lipase |
Serum amylase |
||||
| Prospective study [56] |
384/60 |
Two times URL |
Diagnostic accuracy and efficiency are > 95% for both |
No difference between amylase and lipase in diagnosing AP |
|
| Prospective study [57] |
306/48 |
Serum lipase > 208 U/L Serum amylase > 110 U/L |
92% sensitivity 87% specificity 94% Diagnostic accuracy |
93% sensitivity 87% specificity 91% Diagnostic accuracy |
Both tests are associated with AP, but serum lipase is better than amylase |
| Prospective study [58] |
328/51 |
Serum lipase: > 208 U/L (Day 1) > 216 U/L (Day 3) Serum amylase: > 176 U/L > 126 U/L (Day 3) |
Day 1: 64 % Sensitivity 97% Specificity Day 3: 55% Sensitivity 84% Specificity |
Day 1: 45 % Sensitivity 97% Specificity Day 3: 35% Sensitivity 92% Specificity |
Serum lipase is better at diagnosing early and late AP |
| Retrospective study [63] |
17,531/320 *49 had elevated lipase only |
Serum lipase > 208 U/L Serum amylase > 114 U/L |
90.3% Sensitivity 93.6% Specificity |
78.7% Sensitivity 92.6% Specificity |
Serum lipase is more accurate marker for AP |
| Cohort study [2] |
1,520/44 |
Three times URL |
64% Sensitivity 97% Specificity |
50% Sensitivity 99% Specificity |
Serum lipase is preferable to use in comparison to amylase alone or both tests |
| Retrospective study [59] |
3451/34 *33 patients had elevated amylase and 50 had elevated lipase only |
Three or more times URL |
95.5% Sensitivity 99.2% Specificity |
63.6% Sensitivity 99.4% Specificity |
Both enzymes have good accuracy, but lipase is more sensitive than amylase |
| Cohort study [60] |
151/117 *6 patients with gallstone-induced and 5 patients with alcohol-induced AP had elevated lipase only |
Three times URL |
96.6% Sensitivity 99.4% Specificity |
78.6% Sensitivity 99.1% Specificity |
Lipase is more sensitive in diagnosing AP and using it alone would present a substantial cost saving on health care system |
| Prospective study [61] |
476/154 *58 patients had a normal amylase level |
Three times URL |
91% Sensitivity 92% Specificity |
62% Sensitivity 93% Specificity |
Lipase is more sensitive than amylase and should replace amylase in diagnosis of AP |
| Cohort study [62] |
50/42 *8 patients had elevated lipase only |
Three times URL |
100% Sensitivity |
78.6% Sensitivity |
Lipase is a better choice than amylase in diagnosis of AP |
This table is a list of individual studies examining the specificity and sensitive of serum lipase and serum amylase in diagnosing AP. In each of the listed studies except one, the authors concluded that serum lipase is better than serum amylase for AP. The only outlier used a lower threshold in considering enzyme elevation; in particular, two times the upper limit of reference interval (URL) was used whereas the Atlanta classification system recommends at least three times the URL to determine enzyme elevation (Ismail & Bhayana, 2017).37
Table 2: Summary of studies exploring the cost implication associated with eliminating amylase test.
| Design and Reference |
Costs |
Volume of test |
Results |
| Cohort study (UK) [2] |
Amylase costs £1.94 Lipase cost £2.50 |
1383 request for 62 days costing £6136 for both tests |
Testing lipase only will result in cost saving |
| Cohort study (UK) [60] |
Single amylase or lipase cost about £0.69 each Cost of both measured together were £0.99. |
2979 requests costing £2949.21 |
Measuring lipase would save health care system an estimate of £893.70 per year |
| Prospective study (US) [71] |
Patients charged $35 for either lipase or amylase |
618 co-ordered both lipase and amylase |
Amylase test was removed from common order sets in the electronic medical record Reduced the co-ordering of lipase and amylase to 294 Overall saving of $135,000 per year |
This table specifically outlines studies that compared the financial cost of the serum amylase and serum lipase tests for diagnosing AP. All three studies show cost savings if only lipase concentration is used. In fact, one study by researchers in Pennsylvania resulted in the removal of the amylase test “from common order sets in the electronic medical record.”37
Furey, et al. (2020) compared amylase and lipase ordering patterns for patients with AP. A total of 438 individuals were included in this study. The researchers noted that “All patients had at least one lipase ordered during their admission, and only 51 patients (12%) had at least one amylase ordered. On average, lipase was elevated 5 times higher above its respective upper reference limit than amylase at admission.”42 Further, patients undergoing a laparoscopic cholecystectomy (gallbladder removal) were more likely to have amylase ordered. These results showed that in 88% of patients with AP, amylase measurement was not necessary; moreover, “Of patients for whom amylase was ordered, it was common for these patients to be those referred to surgical procedures, possibly because amylase normalization may be documented faster than that of lipase.”42
In a retrospective cross-sectional study by El Halabi, et al. (2019), the clinical utility and economic burden of routine serum lipase examination in the emergency department was observed. From 24,133 adult patients admitted within a 12-month period, serum lipase levels were ordered for 4,976 (20.6%) patients. Of those 614 (12.4%) who had abnormal lipase levels, 130 of those patients were above the diagnostic threshold for AP (>3 times the ULN) and 75 patients had confirmed diagnosis of acute pancreatitis. In total, 1,890 patients had normal no abdominal pain or history of acute pancreatitis, but 251 of these patients were tested for lipase levels, leading to a total cost of $51,030. These results triggered unneeded cross-sectional abdominal imaging in 61 patients and unwarranted gastroenterology consultation in three patients, leading to an additional charge of $28,975. The authors conclude that "serum lipase is widely overutilised in the emergency setting resulting in unnecessary expenses and investigations.”43
Liu, et al. (2021) studied the use of serum amylase and lipase for the prediction of pancreatic injury in critically ill children admitted to the PICU. Seventy-nine children who died from different cases were studied from autopsy and it was found that 41 of these patients had pathological signs of pancreatic injury. Multivariable logistic regression analysis showed that serum amylase, serum lipase, and septic shock were significantly associated with the occurrence rate of pancreatic injury. Serum amylase was measured with 53.7% sensitivity, 81.6% specificity, cut off value of 97.5, and AUC of 0.731. Serum lipase was measured with 36.6% sensitivity, 92.1% specificity, cut off value of 61.1, and AUC of 0.727. The authors conclude that “serum amylase and lipase could serve as independent biomarkers to predict pancreatic injury in critically ill children.”32
Ritter. J, et al. (2019) showed that for individuals with AP experiencing a hospital stay, there was no difference in disease severity between individuals who had repeat lipase and/or amylase testing and those who did not have repeat testing. They found that approximately “one-third of inpatient encounters with at least one elevated amylase or lipase test continued with repeat testing with as many as 25 additional tests after the initial elevated test result. The mean number of unnecessary additional serial tests was 2.8 and 2.4 for amylase and lipase, respectively, consistent with the tests being ordered each hospital day, given a 3-day nationwide average inpatient stay for acute pancreatitis.”44 According to their findings, “ambulatory settings had the highest rates of concurrent testing while emergency departments had the lowest.”44 While the cost of unnecessary serial and concurrent amylase/lipase tests are relatively small when considering the entire health system, based on their findings, they estimated that the national impact of these two tests could be as much as $5.8 million in variable costs alone. They concluded that unnecessary laboratory testing remains a problem despite evidence-based guidelines and programs that have been designed to reduce and eliminate it.44
Trypsin/Trypsinogen/TAP
Trypsin is a protease produced by the pancreatic acinar cells. Trypsin is first synthesized in its zymogen form, trypsinogen, which has its N-terminus cleaved to form the mature trypsin. Pancreatitis can result in blockage of the release of the proteases while their synthesis continues. This increase in both intracellular trypsinogen and cathepsin B, an enzyme that can cleave the trypsinogen activation peptide (TAP) from the zymogen to form mature trypsin, results in a premature intrapancreatic activation of trypsin. This triggers a release of both trypsin and TAP extracellularly into the serum and surrounding peripancreatic tissue. Due to the proteolytic nature of trypsin, this response can result in degradation of both the pancreatic and peripancreatic tissues (i.e., necrotizing AP).19,45 Trypsin activity “is critical for the severity of both acute and chronic pancreatitis.”46 When the intracellular activity of trypsin escalates, an increase is also reflected in the number of pancreatitis cases overall, as well as in the severity of these cases.47
Since trypsinogen is readily excreted, a urine trypsinogen-2 dipstick test has been developed (Actim Pancreatitis test strip from Medix Biochemica), which has a reported specificity of 85% for severe AP within 24 hours of hospital admission.48 Another study reported that the trypsinogen-2 dipstick test has a specificity of 95% and a sensitivity of 94% for AP, which is higher than a comparable urine test for amylase.49 As of 2023, the FDA has not approved the use of the trypsinogen-2 dipstick test for the detection or diagnosis of AP. The quality control review of the clinical trial is underway in the United States.50 The use of TAP for either a diagnostic or prognostic tool is of debate.29
The study by Neoptolemos, et al. (2000) reported that a urinary TAP assay had a 73% specificity for AP. However, another study using a serum TAP methodology reported a 23.5% sensitivity and 91.7% specificity for AP and concluded that “TAP is of limited value in assessing the diagnosis and the severity of acute pancreatic damage.”52
Yasuda, et al. (2019) completed a multicenter study in Japan which measured the usefulness of the rapid urinary trypsinogen-2 dipstick test and levels of urinary trypsinogen-2 and TAP concentration as prognostic tools for AP. A total of 94 patients participated in this study from 17 medical institutions between April 2009 and December 2012. The researchers determined that “The trypsinogen-2 dipstick test was positive in 57 of 78 patients with acute pancreatitis (sensitivity, 73.1%) and in 6 of 16 patients with abdominal pain but without any evidence of acute pancreatitis (specificity, 62.5%).” Further, both TAP and urinary trypsinogen-2 levels were significantly higher in patients with extra-pancreatic inflammation. The authors concluded that the urinary trypsinogen-2 dipstick test is a useful tool for AP diagnoses.53
Simha, et al. (2021) studied the utility of POC urine trypsinogen dipstick test for diagnosing AP in an emergency unit. Urine trypsinogen dipstick test (UTDT) was performed in 187 patients in which 90 patients had AP. UTDT was positive in 61 (67.7%) of the 90 AP patients. In the 97 non pancreatitis cases, UTDT was positive in nine of those cases (9.3%). The sensitivity and specificity of UTDT for AP was 67.8% and 90.7%, respectively. The authors conclude that although it is a great and convenient possibility as a POC test, “the low sensitivity of UTDT could be a concern with its routine use.”54
Allemann, et al. (2024) studied the predictive value of serum trypsin and TAP in the assessment of AP severity. The authors conducted a single center cohort study with 142 patients, and a systematic literature review. In the cohort study, nine patients had severe AP and 81 patients had mild AP. “The ratio of the geometric mean of severe vs. mild AP for trypsin was 0.72 (95% CI: 0.51-1.00), p = 0.053 and, for TAP, 0.74 (95% CI: 0.54-1.01), p = 0.055, respectively.” The literature review had “conflicting results” regarding the predictive value of serum TAP and trypsin. The authors concluded that “Serum TAP and trypsin have an inferior predictive value of severity of AP compared to the clinical APACHE II score.”55
Other Biochemical Markers (CRP, Procalcitonin, IL-6, IL-8)
Acute pancreatitis results in the activation of the immune system. Specific markers including C-reactive protein (CRP), procalcitonin, interleukin-6 (IL-6), and interleukin-8 (IL-8) have been linked to AP.19,56,57 CRP is a nonspecific marker for inflammation that takes 48-72 hours to reach maximal concentration after initial onset of AP but is reported to have a specificity of 93% in detecting pancreatic necrosis. CRP can be used in monitoring the severity of AP; however, imaging techniques, including CT, and evaluative tools, such as the APACHE-II (acute physiology and chronic health evaluation) test, are preferred methods.7,21
Procalcitonin is the inactive precursor of the hormone calcitonin. Like CRP, procalcitonin has been linked to inflammatory responses, especially in response to infections and sepsis. Procalcitonin levels are elevated in AP and are significantly elevated (≥3.5 ng/mL for at least two consecutive days) in cases of AP associated with multiorgan dysfunction syndrome (MODS).58 Moreover, the elevated procalcitonin levels decrease upon treatment for AP; “however, further research is needed in order to understand how these biomarkers can help to monitor inflammatory responses in AP.”59
The concentration of inflammatory cytokines IL-6 and IL-8 become elevated in AP with a maximal peak within the first 24 hours after initial onset of AP.19 One study by Jakkampudi, et al. (2017) shows that IL-6 and IL-8 are released in a time-dependent manner after injury to the pancreatic acinar cells. This, in turn, activated the peripheral blood mononuclear cells (PBMCs), which propagate acinar cell apoptosis that results in further release of cytokines to increase the likelihood of additional cellular damage.
A study conducted by Khanna, et al. (2013) compares the use of biochemical markers, such as CRP, IL-6, and procalcitonin, in predicting the severity of AP and necrosis to that of the clinically used evaluative tools, including the Glasgow score and APACHE-II test. Their results indicate that CRP has a sensitivity and specificity of 86.2% and 100%, respectively, for severe AP and a sensitivity and specificity of 100% and 81.4%, respectively, for pancreatic necrosis. These scores are better than those reported for the clinical evaluative tools (see table below). IL-6 also shows an increase in both sensitivity and specificity; however, the values for procalcitonin are considerably lower than either CRP or IL-6 in all parameters.61
| Data from |
Severe AP |
Pancreatic necrosis |
||
| (Khanna et al., 2013) |
Sensitivity |
Specificity |
Sensitivity |
Specificity |
| Glasgow |
71.0 |
78.0 |
64.7 |
63.6 |
| APACHE-II |
80.6 |
82.9 |
64.7 |
61.8 |
| CRP |
86.2 |
100 |
100 |
81.4 |
| IL-6 |
93.1 |
96.8 |
94.1 |
72.1 |
| Procalcitonin |
86.4 |
75.0 |
78.6 |
53.6 |
Another study by Hagjer and Kumar (2018) compared the efficacy of the bedside index for severity in AP (BISAP) scoring system to CRP and procalcitonin shows that CRP is not as accurate for prognostication as BISAP. BISAP has AUCs for predicting severe AP and death of 0.875 and 0.740, respectively, as compared to the scores of CRP (0.755 and 0.693, respectively). Procalcitonin, on the other hand, had values of 0.940 and 0.769 for predicting severe AP and death, respectively. The authors concluded that it “is a promising inflammatory marker with prediction rates similar to BISAP.”62
Li, et al. (2018) completed a meta-analysis to determine the relationship between high mobility group box 1 (HMGB1), interleukin-6 (IL-6) and AP. HMGB1 protein is a nuclear protein with several different purposes depending on its location.64 These researchers analyzed data from 27 different studies comprised of 1908 of participants (896 with mild AP, 700 with severe AP and 312 healthy controls). Overall, serum HMGB1 and IL-6 levels were higher in patients with both severe and mild AP compared to controls; further, and serum HMGB1 and IL-6 levels were significantly higher in patients with severe AP than mild AP.63 The authors concluded that serum HMGB1 and IL-6 levels “might be used as effective indicators for pancreatic lesions as well as the degree of inflammatory response” and that both HMGB1 and IL-6 are closely correlated with pancreatitis severity.
Tian, et al. (2020) studied the diagnostic value of C-reactive protein (CRP), procalcitonin (PCT), IL-6, and lactate dehydrogenase (LDH) in patients with severe acute pancreatitis. A total of 153 patients were divided into the mild AP group (81) and severe pancreatitis group (72). Significant differences in the values of these enzymes were found between both groups. The sensitivity, specificity, and AUC were determined as seen in the chart below. The AUC of combined detection of CRP, PCT, IL-6 and LDH was 0.989. The authors conclude that "the combined detection of CRP, PCT, IL-6 and LDH has a high diagnostic value for judging the severity of acute pancreatitis.”65
| Enzyme |
Sensitivity |
Specificity |
AUC |
| CRP |
55.6% |
73% |
0.637 |
| PCT |
77.8% |
94% |
0.929 |
| IL-6 |
80.2% |
85% |
0.886 |
| LDH |
82.7% |
96% |
0.919 |
In a retrospective cohort study, Wei, et al. (2022) investigated the predictive value of serum cholinesterase (ChE) in the mortality of acute pancreatitis. A total of 692 patients were enrolled in the study and were divided into the ChE-low group (378 patients) or ChE-normal group (314 patients). Mortality was significantly different in two groups (10.3% in ChE-low vs. 0.0% ChE- normal) and organ failure also differed (46.6% ChE-low vs. 8.6% ChE-normal). The area under the curve of serum ChE was 0.875 and 0.803 for mortality and organ failure, respectively. The authors conclude that "lower level of serum ChE was independently associated with the severity and mortality of AP.”66
Wu, et al. (2024) studied the predictive ability of CRP for AP. The authors conducted a meta-analysis of 41 studies including 6154 AP cases. The authors calculated a summary operating characteristic curve to measure the diagnostic value of CRP. The area under the curve was 0.85, the sensitivity was 0.76, and the specificity was 0.79. The authors concluded that “CRP has significant value as a biomarker for assessing AP severity.”67
International Association of Pancreatology (IAP/APA Working Group) and the American Pancreatic Association (APA)
In 2012, a joint conference between the IAP and the APA convened to address the guidelines for the management of acute pancreatitis. This conference made their recommendations using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. The IAP/APA Working Group (2013) are detailed with 38 recommendations covering 12 different topics, ranging from diagnosis to predicting severity of disease to timing of treatments. As concerning the diagnosis and etiology of AP, the associations conclude with “GRADE 1B, strong agreement” that the definition of AP follow the Atlanta classification system where at least two of the following three criteria are evident—the clinical manifestation of upper abdominal pain, the laboratory testing of serum amylase or serum lipase where levels are more than three times the upper limit of normal values, and/or the affirmation of pancreatitis using imaging methods.7 IAP/APA Working Group (2013) specifically did not include the trypsinogen-2 dipstick test in their recommendations “because of its presumed limited availability.” One question addressed by the committee was the continuation of oral feeding being withheld for patients until the lab serum tests returned within normal values. With a GRADE 2B, strong agreement finding, they conclude that “it is not necessary to wait until pain or laboratory abnormalities completely resolve before restarting oral feeding.”7 No specific discussion on the preference of either serum amylase or lipase is included within the guidelines as well as no discussion of the use of either serum test beyond initial diagnosis of AP (i.e., no continual testing for disease monitoring is included). Furthermore, no discussion concerning the use of urinary or peritoneal amylase concentrations for AP.
With regards to CRP and/or procalcitonin, the IAP/APA does not address the topic in any detail. As part of IAP/APA Working Group (2013) recommendation (GRADE 2B) concerning the best score or marker to predict the severity of AP, they state “that there are many different predictive scoring systems for acute pancreatitis..., including single serum markers (C-reactive protein, hematocrit, procalcitonin, blood urea nitrogen), but none of these are clearly superior or inferior to (persistent) SIRS”, which is Systemic inflammatory response syndrome. Moreover, in response to their recommendation for admission to an intensive care unit in AP (GRADE 1C), they state that “the routine use of single markers, such as CRP, hematocrit, BUN or procalcitonin alone to triage patients to an intensive care setting is not recommended.”7
American Gastroenterological Association (AGA)
The Clinical Practice and Economics Committee (CPEC) of the American Gastroenterological Association (AGA) Institute released the AGA Institute Medical Position Statement on Acute Pancreatitis as approved by the AGA Institute Governing Board in 2007 to address differences in the recommendations of various national and international societies concerning AP. Within their recommendations, Baillie (2007) address the necessity of timeliness in the applicability of serum amylase and/or serum lipase testing. Per their recommendations, either serum amylase or serum lipase should be tested within 48 hours of admission. AP is consistent with amylase or lipase levels greater than three times the upper limit of the normal value. Baillie (2007) specifically state that the “elevation of lipase levels is somewhat more specific and is thus preferred.” The AGA guidelines do not address the use of either urinary or peritoneal concentrations of amylase in AP. Also, any patient presenting symptoms of unexplained multiorgan failure or systemic inflammatory response syndrome should be tested for a possible AP diagnosis. Concerning etiology of the phenotype, they suggest that upon admission, “all patients should have serum obtained for measurement of amylase or lipase level, triglyceride level, calcium level, and liver chemistries.”68 Invasive evaluation, such as endoscopic retrograde cholangiopancreatography (ERCP), should be avoided for patients with a single occurrence of AP. The only mention of CRP in their guidelines is in the section concerning the severity (and not the diagnosis of) AP. “Laboratory tests may be used as an adjunct to clinical judgment, multiple factors scoring systems, and CT to guide clinical triage decisions. A serum C-reactive protein level >150 mg/L at 48 hours after disease onset is preferred.”68
In 2018, the AGA published guidelines on the initial management of AP. These guidelines state that “the diagnosis of AP requires at least 2 of the following features: characteristic abdominal pain; biochemical evidence of pancreatitis (ie, amylase or lipase elevated >3 times the upper limit of normal); and/or radiographic evidence of pancreatitis on cross-sectional imaging.”69
The AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatic Insufficiency (EPI) advise that exocrine pancreatic insufficiency “should be suspected in patients with high-risk clinical conditions, such as chronic pancreatitis, relapsing acute pancreatitis, pancreatic ductal adenocarcinoma, cystic fibrosis, and previous pancreatic surgery. . . fecal elastase test is the most appropriate initial test and must be performed on a semi-solid or solid stool specimen. A fecal elastase level <100 μg/g of stool provides good evidence of EPI, and levels of 100–200 μg/g are indeterminate for EPI.”70
American College of Gastroenterology (ACG)
The ACG released guidelines concerning AP in 2006 and 2024. Both sets of guidelines recommend the use of the Atlanta classification system regarding the threshold of either serum amylase or serum lipase levels in the diagnosis of AP (i.e., greater than three times the upper limit of normal range). Both sets of guideline’s state that the standard diagnosis is meeting at least two of the three criteria as stated in the revised Atlanta classification system.8,9
The 2006 guidelines discuss the differences between serum amylase and lipase in greater detail. First, although both enzymes can be elevated in AP, the sensitivity and half-life of lipase are more amenable for diagnosis since the levels of lipase remain elevated longer than those of amylase. These guidelines also make note that “it is usually not necessary to measure both serum amylase and lipase” and that “the daily measurement of serum amylase or lipase after the diagnosis of acute pancreatitis has limited value in assessing the clinical progress of the illness.” These guidelines discuss the possibility of elevated amylase levels due to causes other than AP, including but not limited to macroamylasemia, whereas the serum levels of lipase are unaffected by these conditions.8
In 2024 the ACG published guidelines on the management of acute pancreatitis. The guidelines state that “Due to limitations on sensitivity and negative predictive value, serum amylase alone cannot be used reliably for the diagnosis of AP, and serum lipase is preferred.” The guidelines explain that “Serum lipase seems to be more specific and remains elevated longer than amylase following disease presentation.”71
The 2024 guidelines state that “Although numerous laboratory tests have been studied to predict severity in patients with AP, no single laboratory test is consistently accurate to predict severity in patients with AP.” The guidelines not that “While many studies, especially from Europe, have used the acute-phase reactant C-reactive protein to determine severity, it is not practical because it takes 48–72 hours to become accurate in predicting necrosis and/or death. By that time, most patients have already developed obvious mild or severe disease.” The guidelines also point out that “Several investigators have found a rise in HCT and/or rising BUN at 24 hours to be a reliable test in predicting mortality and persisting multiorgan failure in patients with AP.”71
The 2024 guidelines state that, during assessment of the etiology of AP, “In the absence of gallstones and/or a significant history of alcohol use, serum triglyceride (TG) should be obtained and considered the etiology, preferably if greater than 1,000 mg/dL.”71
American Board of Internal Medicine (ABIM), American Society for Clinical Pathology (ASCP), and Choosing Wisely
In 2020, the ASCP, along with Choosing Wisely and the ABIM Foundation, published a brochure titled Thirty Things Physicians and Patients Should Question. This brochure includes the following recommendation
“Do not test for amylase in cases of suspected acute pancreatitis. Instead, test for lipase.
Amylase and lipase are digestive enzymes normally released from the acinar cells of the exocrine pancreas into the duodenum. Following injury to the pancreas, these enzymes are released into the circulation. While amylase is cleared in the urine, lipase is reabsorbed back into the circulation. In cases of acute pancreatitis, serum activity for both enzymes are greatly increased.
Serum lipase is now the preferred test due to its improved sensitivity, particularly in alcohol-induced pancreatitis. Its prolonged elevation creates a wider diagnostic window than amylase. In acute pancreatitis, amylase can rise rapidly within 3–6 hours of the onset of symptoms and may remain elevated for up to five days. Lipase, however, usually peaks at 24 hours with serum concentrations remaining elevated for 8–14 days. This means it is far more useful than amylase when the clinical presentation or testing has been delayed for more than 24 hours.
Current guidelines and recommendations indicate that lipase should be preferred over total and pancreatic amylase for the initial diagnosis of AP and that the assessment should not be repeated over time to monitor disease prognosis. Repeat testing should be considered only when the patient has signs and symptoms of persisting pancreatic or peripancreatic inflammation, blockage of the pancreatic duct or development of a pseudocyst. Testing both amylase and lipase is generally discouraged because it increases costs while only marginally improving diagnostic efficiency compared to either marker alone.”72
North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee (NASPGHAN)
The NASPGHAN states that the primary biomarkers used to diagnose AP are serum lipase and amylase and note that “a serum lipase or amylase level of at least 3 times the upper limit of normal is considered consistent with pancreatitis.” Further, NASPGHAN acknowledges that other biomarkers for diagnosis and management of AP have been investigated, but none are prominent and “many have yet to be validated for general clinical use.”73
The NASPGHAN defines the diagnosis of AP as “Requires at least 2 of the following 1) Abdominal pain not attributable to another cause or suggestive of AP 2) Amylase or lipase ≥ 3x the upper limits of normal 3) Imaging consistent with pancreatitis.”74
American Psychiatric Association
The American Psychiatric Association published a practice guideline in 2023 for the treatment of patients with eating disorders. In this guideline, pancreatitis (in adults and in adolescents) is just one of a set of factors that supports medical hospitalization or hospitalization on a specialized eating disorder unit.75
The American Psychiatric Association notes that “serum amylase levels, specifically levels of salivary amylase, may be elevated in patients who self-induce vomiting. With starvation and with renourishment, elevations in serum lipase can be seen but generally do not require intervention.”75
Academy for Eating Disorders (AED) Medical Care Standards Committee
The AED has published a guide to medical care for eating disorders. A table is included in these guidelines which is titled Diagnostic Tests Indicated for All Patients with A Suspected ED [eating disorder]. In a subcategory, titled Criteria Supportive of Hospitalization for Acute Medical
Stabilization, these guidelines mention that “acute medical complications of malnutrition” including pancreatitis may occur.76
The American Association for Clinical Chemistry (AACC)
The American Association for Clinical Chemistry released recommendations for amylase testing in diagnosis and management of acute pancreatitis. The AACC provides the following recommendations:
- “For diagnosis and management of acute pancreatitis, do not order this test if serum lipase test is available.
- May be considered for the diagnosis and monitoring of chronic pancreatitis and other pancreatic diseases.”
The AACC does mention that “the test is not specific for pancreatitis and may be elevated due to other, non-pancreatic causes (such as acute cholecystitis, inflammatory bowel disease, intestinal obstruction, certain cancers, salivary disease, macroamylasemia, etc.).”
The AACC further states to “consider ordering this test when serum lipase is not available as a stat test and the patient presents with a sudden onset of abdominal pain with nausea and vomiting, fever, hypotension, and abdominal distension” and that “testing both amylase and lipase should be discouraged because it increases costs while only marginally improving diagnostic efficiency compared to lipase alone.”77
Canadian Agency for Drugs and Technologies in Health (CADTH)
The CADTH has published an advisory panel guidance on minimum retesting intervals for lab tests. They identify the following key issues:
- “Lab test overuse can contribute to further unnecessary follow-up and testing, negative patient experiences, potentially inappropriate treatments, and the inefficient use of health care resources. One review of lab testing in Canada found that around 22% of blood tests were likely unnecessary.
- One strategy to address lab test overuse is to establish minimal retesting intervals that can be implemented in medical laboratories to help identify and manage potentially inappropriate lab test requests.
- Minimum retesting intervals suggest the minimum time before a test should be repeated based on the biochemical properties of the test and the clinical situation in which it is used. They are intended to inform clinical decisions about repeat testing.”78
Specific to repeat lipase testing, they do not recommend reordering lipase tests:
- “Do not reorder lipase tests for monitoring patients with an established diagnosis of acute pancreatitis.
- Do not reorder lipase tests for monitoring patients with an established diagnosis of chronic pancreatitis.
An exception to this recommendation is if there is clinical suspicion of acute-on-chronic pancreatitis, where lipase testing is required for diagnostic purposes.”78
Implementation advise for this recommendation: “To support reductions in unnecessary retesting, in outpatient or community settings, labs may consider implementing a 6-month hard stop minimum retesting interval.
This recommendation is based on the experience of the advisory panel as no relevant information for serum lipase retesting for chronic pancreatitis was identified in the literature review.”78
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013-01-01 00:00:00 2013;62:102-111. doi:10.1136/gutjnl-2012-302779
- Stevens T, Conwell DL. Exocrine pancreatic insufficiency. Updated November 8, 2023. https://www.uptodate.com/contents/exocrine-pancreatic-insufficiency
- NIDDK. Symptoms & Causes of Pancreatitis. Updated November, 2017. https://www.niddk.nih.gov/health-information/digestive-diseases/pancreatitis/symptoms-causes
- Gapp J, Tariq A, Chandra S. Acute Pancreatitis. StatPearls. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK482468/
- informedhealth.org. Acute pancreatitis: Learn More – How is acute pancreatitis treated? Updated May 25, 2021. https://www.informedhealth.org/acute-pancreatitis.html
- Bradley E. A clinically based classification system for acute pancreatitis: Summary of the international symposium on acute pancreatitis, atlanta, ga, september 11 through 13, 1992. Archives of Surgery. 1993;128(5):586-590. doi:10.1001/archsurg.1993.01420170122019
- IAP/APA Working Group. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4):e1-e15. doi:10.1016/j.pan.2013.07.063
- Banks P, Freeman M. Practice Guidelines in Acute Pancreatitis. Practice Guideline. The American Journal Of Gastroenterology. 2006;101:2379. doi:10.1111/j.1572-0241.2006.00856.x
- Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. Sep 2013;108(9):1400-15; 1416. doi:10.1038/ajg.2013.218
- Bollen TL, Hazewinkel M, Smithuis R. Acute Pancreatitis 2012 Revised Atlanta Classification of Acute Pancreatitis. Radiology Society of the Netherlands. https://radiologyassistant.nl/abdomen/pancreas/acute-pancreatitis
- Freedman SD, Forsmark CE. Chronic pancreatitis: Clinical manifestations and diagnosis in adults. Updated March 3, 2025. https://www.uptodate.com/contents/chronic-pancreatitis-clinical-manifestations-and-diagnosis-in-adults
- Klochkov AK, Pujuitha, Lim Y, Sun Y. Alcoholic Pancreatitis. StatPearls. 2023. https://www.ncbi.nlm.nih.gov/books/NBK537191/
- Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. Apr 2007;132(4):1557-73. doi:10.1053/j.gastro.2007.03.001
- Barry K. Chronic Pancreatitis: Diagnosis and Treatment. American Academy of Family Physician. 2018;Volume 97(Number 6)
- Borowitz D, Grant R, Durie P. Pancreatic Enzymes Clinical Care Guidelines. Cystic Fibrosis Foundation. https://www.cff.org/Care/Clinical-Care-Guidelines/Nutrition-and-GI-Clinical-Care-Guidelines/Pancreatic-Enzymes-Clinical-Care-Guidelines/
- Patel J, Madan, A., Gammon, A., Sossenheimer, M., Samadder, N. J. Rare hereditary cause of chronic pancreatitis in a young male: SPINK1 mutation. The Pan African medical journal. 2017;28:110. doi:10.11604/pamj.2017.28.110.13854
- Basnayake C, Ratnam D. Blood tests for acute pancreatitis. Australian Prescriber. 08/03 2015;38(4):128-130. doi:10.18773/austprescr.2015.043
- Vege SS. Approach to the patient with elevated serum amylase or lipase. Updated September 11, 2024. https://www.uptodate.com/contents/approach-to-the-patient-with-elevated-serum-amylase-or-lipase
- Yadav D, Agarwal N, Pitchumoni CS. A critical evaluation of laboratory tests in acute pancreatitis. Am J Gastroenterol. Jun 2002;97(6):1309-18. doi:10.1111/j.1572-0241.2002.05766.x
- Ventrucci M, Pezzilli R, Naldoni P, et al. Serum pancreatic enzyme behavior during the course of acute pancreatitis. Pancreas. 1987;2(5):506-9. doi:10.1097/00006676-198709000-00003
- Quinlan JD. Acute pancreatitis. American family physician. Nov 1 2014;90(9):632-9.
- Clavien PA, Robert J, Meyer P, et al. Acute pancreatitis and normoamylasemia. Not an uncommon combination. Annals of surgery. Nov 1989;210(5):614-20. doi:10.1097/00000658-198911000-00008
- Liu S, Wang Q, Zhou R, et al. Hyperamylasemia as an Early Predictor of Mortality in Patients with Acute Paraquat Poisoning. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. 2016;22:1342-1348. doi:10.12659/msm.897930
- Ceylan ME, Evrensel A, Önen Ünsalver B. Hyperamylasemia Related to Sertraline. Korean Journal of Family Medicine. 2016;37(4):259-259. doi:10.4082/kjfm.2016.37.4.259
- Wolfe BE, Jimerson DC, Smith A, Keel PK. Serum Amylase in Bulimia Nervosa and Purging Disorder: Differentiating the Association with Binge Eating versus Purging Behavior. Physiology & behavior. 07/18 2011;104(5):684-686. doi:10.1016/j.physbeh.2011.06.025
- Herrmann-Storck C, Saint Louis M, Foucand T, et al. Severe Leptospirosis in Hospitalized Patients, Guadeloupe. Emerging Infectious Diseases. 2010;16(2):331-334. doi:10.3201/eid1602.090139
- Terui K, Hishiki T, Saito T, Mitsunaga T, Nakata M, Yoshida H. Urinary amylase / urinary creatinine ratio (uAm/uCr) - a less-invasive parameter for management of hyperamylasemia. BMC Pediatrics. 2013;13:205-205. doi:10.1186/1471-2431-13-205
- Kumar P, Ghosh A, Tandon V, Sahoo R. Gullo’s Syndrome: A Case Report. Journal of Clinical and Diagnostic Research : JCDR. 2016;10(2):OD21-OD22. doi:10.7860/jcdr/2016/17038.7285
- Lippi G, Valentino M, Cervellin G. Laboratory diagnosis of acute pancreatitis: in search of the Holy Grail. Critical Reviews in Clinical Laboratory Sciences. 2012/02/01 2012;49(1):18-31. doi:10.3109/10408363.2012.658354
- Rompianesi G, Hann A, Komolafe O, Pereira SP, Davidson BR, Gurusamy KS. Serum amylase and lipase and urinary trypsinogen and amylase for diagnosis of acute pancreatitis. Cochrane Database of Systematic Reviews. 2017;(4)doi:10.1002/14651858.cd012010.pub2
- Burkart J, Haigler S, Caruana R, Hylander B. Usefulness of peritoneal fluid amylase levels in the differential diagnosis of peritonitis in peritoneal dialysis patients. Journal of the American Society of Nephrology : JASN. Apr 1991;1(10):1186-90. doi:10.1681/asn.v1101186
- Liu P, Xiao Z, Yan H, et al. Serum Amylase and Lipase for the Prediction of Pancreatic Injury in Critically Ill Children Admitted to the PICU. Pediatric Critical Care Medicine. 2021;22(1):e10-e18. doi:10.1097/pcc.0000000000002525
- Judal H, Ganatra V, Choudhary PR. Urinary amylase levels in the diagnosis of acute pancreatitis: a prospective case control study. International Surgery Journal. 2022;9(2):432-437. doi:10.18203/2349-2902.isj20220337
- Ryholt V, Soder J, Enderle J, Rajendran R. Assessment of appropriate use of amylase and lipase testing in the diagnosis of acute pancreatitis at an academic teaching hospital. Lab Med. Feb 22 2024;doi:10.1093/labmed/lmae008
- Mogekar S, Jayakar S, Sri Sai Teja Sampath K, Badangi V. A Study on Urinary Amylase and Serum Amylase in Diagnosing Acute Pancreatitis. Cureus. Oct 2024;16(10):e70809. doi:10.7759/cureus.70809
- Coffey MJ, Nightingale S, Ooi CY. Serum Lipase as an Early Predictor of Severity in Pediatric Acute Pancreatitis. Journal of Pediatric Gastroenterology and Nutrition. 2013;56(6):602-608. doi:10.1097/mpg.0b013e31828b36d8
- Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. Dec 2017;50(18):1275-1280. doi:10.1016/j.clinbiochem.2017.07.003
- Levy P, Boruchowicz A, Hastier P, et al. Diagnostic criteria in predicting a biliary origin of acute pancreatitis in the era of endoscopic ultrasound: multicentre prospective evaluation of 213 patients. Pancreatology. 2005;5(4-5):450-6. doi:10.1159/000086547
- Gumaste VV, Dave PB, Weissman D, Messer J. Lipase/amylase ratio. A new index that distinguishes acute episodes of alcoholic from nonalcoholic acute pancreatitis. Gastroenterology. Nov 1991;101(5):1361-6. doi:10.1016/0016-5085(91)90089-4
- Tenner SM, Steinberg W. The admission serum lipase:amylase ratio differentiates alcoholic from nonalcoholic acute pancreatitis. Am J Gastroenterol. Dec 1992;87(12):1755-8.
- Pacheco RC, Oliveira LC. [Lipase/amylase ratio in biliary acute pancreatitis and alcoholic acute/acutized chronic pancreatitis]. Arquivos de gastroenterologia. Jan-Mar 2007;44(1):35-8. Relacao lipase/amilase nas pancreatites agudas de causa biliar e nas pancreatites agudas/cronicas agudizadas de causa alcoolica. doi:10.1590/s0004-28032007000100008
- Furey C, Buxbaum J, Chambliss AB. A review of biomarker utilization in the diagnosis and management of acute pancreatitis reveals amylase ordering is favored in patients requiring laparoscopic cholecystectomy. Clin Biochem. Mar 2020;77:54-56. doi:10.1016/j.clinbiochem.2019.12.014
- El Halabi M, Bou Daher H, Rustom LBO, et al. Clinical utility and economic burden of routine serum lipase determination in the Emergency Department. International Journal of Clinical Practice. 2019;73(12):e13409. doi:10.1111/ijcp.13409
- Ritter. J, Ghirimoldi. F, Manuel. L, et al. Cost of Unnecessary Amylase and Lipase Testing at Multiple Academic Health Systems. 2019;doi:10.1093/ajcp/aqz170
- Vege SS. Pathogenesis of acute pancreatitis. Updated September 11, 2024. https://www.uptodate.com/contents/pathogenesis-of-acute-pancreatitis
- Zhan X, Wan J, Zhang G, et al. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. Jun 1 2019;316(6):G816-g825. doi:10.1152/ajpgi.00004.2019
- Sendler M, Lerch MM. The Complex Role of Trypsin in Pancreatitis. Gastroenterology. Mar 2020;158(4):822-826. doi:10.1053/j.gastro.2019.12.025
- Lempinen M, Kylänpää-Bäck M-L, Stenman U-H, et al. Predicting the Severity of Acute Pancreatitis by Rapid Measurement of Trypsinogen-2 in Urine. Clinical Chemistry. 2001;47(12):2103. doi:10.1093/clinchem/47.12.2103
- Kemppainen EA, Hedstrom JI, Puolakkainen PA, et al. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. The New England journal of medicine. Jun 19 1997;336(25):1788-93. doi:10.1056/nejm199706193362504
- Eastler J. Urine Trypsinogen 2 Dipstick for the Early Detection of Post-ERCP Pancreatitis. National Library of Medicine-National Institutes of Health. https://clinicaltrials.gov/study/NCT03098082
- Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet (London, England). Jun 3 2000;355(9219):1955-60. doi:10.1016/S0140-6736(00)02327-8
- Pezzilli R, Venturi M, Morselli-Labate AM, et al. Serum Trypsinogen Activation Peptide in the Assessment of the Diagnosis and Severity of Acute Pancreatic Damage: A Pilot Study Using a New Determination Technique. Pancreas. 2004;29(4):298-305. doi:10.1097/00006676-200411000-00009
- Yasuda H, Kataoka K, Takeyama Y, et al. Usefulness of urinary trypsinogen-2 and trypsinogen activation peptide in acute pancreatitis: A multicenter study in Japan. World J Gastroenterol. Jan 7 2019;25(1):107-117. doi:10.3748/wjg.v25.i1.107
- Simha A, Saroch A, Pannu AK, et al. Utility of point-of-care urine trypsinogen dipstick test for diagnosing acute pancreatitis in an emergency unit. Biomarkers in Medicine. 2021;15(14):1271-1276. doi:10.2217/bmm-2021-0067
- Allemann A, Staubli SM, Nebiker CA. Trypsin and Trypsinogen Activation Peptide in the Prediction of Severity of Acute Pancreatitis. Life (Basel). Aug 23 2024;14(9)doi:10.3390/life14091055
- Vege SS. Clinical manifestations and diagnosis of acute pancreatitis. Updated October 7, 2024. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-acute-pancreatitis
- Toouli J, Brooke-Smith M, Bassi C, et al. Guidelines for the management of acute pancreatitis. Journal of gastroenterology and hepatology. Feb 2002;17 Suppl:S15-39. doi:10.1046/j.1440-1746.17.s1.2.x
- Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Annals of surgery. May 2007;245(5):745-54. doi:10.1097/01.sla.0000252443.22360.46
- Simsek O, Kocael A, Kocael P, et al. Inflammatory mediators in the diagnosis and treatment of acute pancreatitis: pentraxin-3, procalcitonin and myeloperoxidase. Archives of medical science : AMS. Mar 2018;14(2):288-296. doi:10.5114/aoms.2016.57886
- Jakkampudi A, Jangala R, Reddy R, et al. Acinar injury and early cytokine response in human acute biliary pancreatitis. Scientific reports. Nov 10 2017;7(1):15276. doi:10.1038/s41598-017-15479-2
- Khanna AK, Meher S, Prakash S, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surgery. 2013:367581. doi:10.1155%2F2013%2F367581
- Hagjer S, Kumar N. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis - A prospective observational study. International journal of surgery (London, England). Apr 21 2018;54(Pt A):76-81. doi:10.1016/j.ijsu.2018.04.026
- Li N, Wang BM, Cai S, Liu PL. The Role of Serum High Mobility Group Box 1 and Interleukin-6 Levels in Acute Pancreatitis: A Meta-Analysis. J Cell Biochem. Jan 2018;119(1):616-624. doi:10.1002/jcb.26222
- Yang H, Wang H, Chavan SS, Andersson U. High Mobility Group Box Protein 1 (HMGB1): The Prototypical Endogenous Danger Molecule. Mol Med. Oct 27 2015;21 Suppl 1:S6-s12. doi:10.2119%2Fmolmed.2015.00087
- Tian F, Li H, Wang L, et al. The diagnostic value of serum C-reactive protein, procalcitonin, interleukin-6 and lactate dehydrogenase in patients with severe acute pancreatitis. Clinica Chimica Acta. 2020/11/01/ 2020;510:665-670. doi:10.1016/j.cca.2020.08.029
- Wei M, Xie X, Yu X, et al. Predictive value of serum cholinesterase in the mortality of acute pancreatitis: A retrospective cohort study. European Journal of Clinical Investigation. 2022:e13741. doi:10.1111/eci.13741
- Wu H, Liao B, Ji T, Huang J, Ma K, Luo Y. Diagnostic value of CRP for predicting the severity of acute pancreatitis: a systematic review and meta-analysis. Biomarkers. Nov 2024;29(7):494-503. doi:10.1080/1354750x.2024.2415463
- Baillie J. AGA Institute Medical Position Statement on Acute Pancreatitis. Gastroenterology. 2007;132(5):2019-2021. doi:10.1053/j.gastro.2007.03.066
- Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. Mar 2018;154(4):1096-1101. doi:10.1053/j.gastro.2018.01.032
- Whitcomb DC, Buchner AM, Forsmark CE. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatic Insufficiency: Expert Review. Gastroenterology. Nov 2023;165(5):1292-1301. doi:10.1053/j.gastro.2023.07.007
- Tenner S, Vege SS, Sheth SG, et al. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. Mar 1 2024;119(3):419-437. doi:10.14309/ajg.0000000000002645
- ASCP. Thirty Things Physicians and Patients Should Question. https://www.ascp.org/content/docs/default-source/get-involved-pdfs/istp_choosingwisely/2019_ascp-30-things-list.pdf
- NASPGHAN. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. 2018;
- Kenneth N. Acute Pancreatitis (AP). https://d1pij0k2lbf86p.cloudfront.net/wp-content/uploads/2024/11/Acute-Pancreatitis-flash-card-final.pdf
- APA. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Eating Disorders. https://psychiatryonline.org/doi/abs/10.1176/appi.books.9780890424865
- AED. Eatinf Disorders A Guide To Medical Care. https://higherlogicdownload.s3.amazonaws.com/AEDWEB/27a3b69a-8aae-45b2-a04c-2a078d02145d/UploadedImages/Publications_Slider/2120_AED_Medical_Care_4th_Ed_FINAL.pdf
- AACC. AACC's Guide to Lab Test Utilization https://www.aacc.org/advocacy-and-outreach/optimal-testing-guide-to-lab-test-utilization/a-f/amylase
- CADTH. Advisory Panel Guidance on Minimum Retesting Intervals for Lab Tests: Appropriate Use Recommendation. 2024. CADTH Health Technology Review.
Coding Section
| Code | Number | Description |
| CPT | 82150 | Amylase |
| 83519 | Immunoassay for analyte other than infectious agent antibody or infectious agent antigen; quantitative, by radioimmunoassay (e.g., RIA | |
| 83520 | Immunoassay for analyte other than infectious agent antibody or infectious agent antigen; quantitative, not otherwise specified | |
| 83529 (effective 01/01/2022) | Interleukin-6 | |
| 83690 | Lipase | |
| 84145 | Procalcitonin (PCT) | |
| 86140 | C-reactive protein | |
| ICD-10 Diagnoses Codes | F50.00-F50.9 | Anorexia nervosa |
| K56.0, K56.3, K56.7 | Ileus | |
| K85.00-K85.92 | Acute pancreatitis | |
| K86.0 and K86.1 | Chronic Pancreatitis | |
| M79.3 | Panniculitis, unspecified | |
| M79.89 | Subcutaneous nodular fat necrosis | |
| R00.0 | Tachycardia | |
| R03.1 | Nonspecific low blood-pressure reading | |
| R06.02 | shortness of breath | |
| R06.82 | Tachypnea, not elsewhere classified | |
| R07.1 | Chest pain on breathing/Painful respiration | |
| R09.02 | Hypoxemia | |
| R10.10, R10.11 and R10.12 | Upper abdominal pain | |
| R10.13 | Epigastric pain | |
| R10.811 – R10.819 | Tenderness when touching the abdomen | |
| R10.84 | Abdominal pain that feels worse after eating | |
| R10.9 | Abdominal pain that radiates to your back | |
| R11.0 | Nausea | |
| R11.10, R11.11 | Vomiting | |
| R11.2 | Nausea with vomiting, unspecified | |
| R14.0 | Abdominal distention | |
| R17 | Unspecified jaundice | |
| R19.00 - R19.09 | Abdominal swelling | |
| R19.11 | Absent bowel sounds | |
| R19.15 | Other abnormal bowel sounds | |
| R50.9 | Fever, fever of chills, elevated body temp | |
| R61 | Generalized hyperhidrosis | |
| R63.0 | Anorexia | |
| R74.8 | Abnormal levels of other serum enzymes | |
| S39.81XA-S39.81XS | Other specified injuries of abdomen | |
| S39.91XA-S39.91XS | Unspecified injury of abdomen | |
| S30.1XXA-S30.1XXS | Contusion of abdominal wall, Flank ecchymosis, Periumbilical ecchymosis |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 07/23/2025 | Annual review, removing amylase from criteria as it is no longer allowed for diagnosis of acte pancreatitis. Also updating description, table of terminoloy, rationale, and references. |
| 10/11/2024 | Annual review, policy being updated for clarity and consistency. Criteria #6 addresses all issues not covered din the first 5 criteria as being not medically necessary. Also updating Note 1, rationale and references, and table of terminology. |
| 07/29/2024 | Change review date to 10/01/2024. |
| 07/11/2023 | Annual review, no change to policy intent. Updating policy for clarity and consistency. Also updating notes, description, table of terminology, rational and references. |
| 07/26/2022 | Annual review, updating policy to indicate that serum lipase testing is preferred over amylase testing, adding coverage for urine specimen testing to criteria #3, adding not medically necessary statement as coverage statement #5. Also updating description, rationale and references. |
| 12/8/2021 |
Updating policy with 2022 coding. Adding code 83529. No other change made. |
| 07/13/2021 |
Annual review, no change to policy intent. Updating rationale and references. |
| 07/08/2020 |
Annual review, adding verbiage relating to testing asymptomatic individuals during a general exam. Also updating background, guidelines and references. |
| 04/15/2020 |
Interim review, updating coding. No other changes. |
| 07/16/2019 |
Annual review, no change to policy intent. Updating coding. |
| 06/03/2019 |
Updating next review date. No other changes. |
| 05/08/2019 |
Updating next review date. No other changes. |
| 06/06/2018 |
New Policy |